Abstract
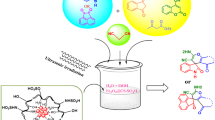
In the present research, a novel, efficient, and green nanocatalyst has been afforded by coating Fe3O4 nanoparticles with chitosan through simple and readily available chemicals. Fourier-transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy, dynamic light scattering, vibrating sample magnetometer, and thermogravimetric analyses were used to describe this nanocatalyst. The catalytic performance of the Fe3O4@chitosan heterogeneous nanocatalyst was investigated in an environmentally benign and efficacious fabrication of a variety of spirooxindole and spirochromene derivatives in high yields via employing three-component reactions of malononitrile, dimedone, and isatin in a solvent-free medium (Method A) and under ultrasonic conditions in EtOH/H2O (Method B) at ambient temperature. The achieved nanocatalyst could be easily removed from the mixture of the reaction and was recyclable seven times via a simple external magnet without appreciable loss in catalytic proficiency. Several other advantages of this methodology were environmental friendliness, simple operation, excellent yields, economical handling, and easy workup.
Graphical abstract
















Similar content being viewed by others
References
N. Rahman, R. Nongkhlaw, Org. Chem. 6, 272–313 (2018). https://doi.org/10.24820/ark
F. Kalantari, A. Ramazani, M.R. Poor Heravi, H. Aghahosseini, K. Ślepokura, Inorg. Chem. 60, 15010–15023 (2021). https://doi.org/10.1021/acs.inorgchem.1c02470
A.K. Rathi, R. Zboril, R.S. Varma, M.B. Gawande, A.C.S. Symp, Series 1238, 39–78 (2016). https://doi.org/10.1021/bk-2016-1238.ch002
M. Narasimhan, M. Chandrasekaran, S. Govindasamy, A. Aravamudhan, J. Environ. Chem. Eng. 9, 104876 (2021). https://doi.org/10.1016/j.jece.2020.104876
S. Liu, B. Yu, S. Wang, Y. Shen, H. Cong, Adv. Colloid Interface Sci. 281, 102165 (2020). https://doi.org/10.1016/j.cis.2020.102165
A. Maleki, F. Hassanzadeh-Afruzi, Z. Varzi, M.S. Esmaeili, Mater. Sci. Eng. C 109, 110502 (2020). https://doi.org/10.1016/j.msec.2019.110502
M. Kamalzare, M.R. Ahghari, M. Bayat, A. Maleki, Sci. Rep. 11, 1–10 (2021). https://doi.org/10.1038/s41598-021-99121-2
M. Dohendou, K. Pakzad, Z. Nezafat, M. Nasrollahzadeh, M.G. Dekamin, Int. J. Biol. Macromol. 192, 771–819 (2021). https://doi.org/10.1016/j.ijbiomac.2021.09.162
A. Maleki, M. Aghaei, N. Ghamari, Chem. Lett. 44, 259–261 (2015). https://doi.org/10.1246/cl.141074
Y.V.D. Nageswar, N.L.C. Domingues, R. Katla, Polysaccharides (2021). https://doi.org/10.1002/9781119711414.ch25
S.S. Khot, P.V. Anbhule, U.V. Desai, P.P. Wadgaonkar, Comptes Rendus Chim. 21, 814–821 (2018). https://doi.org/10.1016/j.crci.2018.05.005
B. Yu, D.Q. Yu, H.M. Liu, Eur. J. Med. Chem. 97, 673–698 (2015). https://doi.org/10.1016/j.ejmech.2014.06.056
K. Ramakumar, T. Maji, J.J. Partridge, J.A. Tunge, Org. Lett. 19, 4014–4017 (2017). https://doi.org/10.1021/acs.orglett.7b01752
N. Ye, H. Chen, E.A. Wold, P.Y. Shi, J. Zhou, A.C.S. Infect, Dis. 2, 382–392 (2016). https://doi.org/10.1021/acsinfecdis.6b00041
M.M. Heravi, T. Momeni, M. Mirzaei, V. Zadsirjan, M. Tahmasebi, Inorg. Nano-Metal. Chem. 51, 896–909 (2021). https://doi.org/10.1080/24701556.2020.1813172
L.M. Zhou, R.Y. Qu, G.F. Yang, Expert Opin. Drug Discov. 15, 603–625 (2020). https://doi.org/10.1080/17460441.2020.1733526
J.P. MacDonald, J.J. Badillo, G.E. Arevalo, A. Silva-García, A.K. Franz, A.C.S. Comb, Sci. 14, 285–293 (2012). https://doi.org/10.1021/co300003c
M. Baghernejad, S. Khodabakhshi, S. Tajik, New J. Chem. 40, 2704–2709 (2016). https://doi.org/10.1039/C5NJ03027G
P. Brandão, C.S. Marques, E.P. Carreiro, M. Pineiro, A.J. Burke, Chem. Rec. 21, 924–1037 (2021). https://doi.org/10.1002/tcr.202000167
M.V. Murlykina, A.D. Morozova, I.M. Zviagin, Y.I. Sakhno, S.M. Desenko, V.A. Chebanov, Front. Chem. 6, 1–43 (2018). https://doi.org/10.3389/fchem.2018.00527
A. Chaudhary, P. Saluja, G. Khanna, Green Chemistry in Environmental Sustainability and Chemical Education (Springer, Singapore, 2018), pp.15–21. https://doi.org/10.1007/978-981-10-8390-7_2
C.J. Gerry, S.L. Schreiber, Curr. Opin. Chem. Biol. 56, 1–9 (2020). https://doi.org/10.1016/j.cbpa.2019.08.008
W.R.J.D. Galloway, A. Isidro-Llobet, D.R. Spring, Nat. Commun. 1, 1–13 (2020). https://doi.org/10.1038/ncomms1081
S. Nagaraju, B. Paplal, K. Sathish, S. Giri, D. Kashinath, Tetrahedron Lett. 58, 4200–4204 (2017). https://doi.org/10.1016/j.tetlet.2017.09.060
S.M. Baghbanian, M. Tajbakhsh, M. Farhang, Comptes Rendus Chim. 17, 1160–1164 (2014). https://doi.org/10.1016/j.crci.2013.12.005
A. Khalafi-Nezhad, S. Mohammadi, A.C.S. Comb, Science 9, 512–518 (2013). https://doi.org/10.1021/co400080z
A. Deepthi, N.V. Thomas, V. Sathi, Curr. Green Chem. 6, 210–225 (2019). https://doi.org/10.2174/2213346106666191019144116
M. Bashkar, M. Bavadi, E. Ghaderi, K. Niknam, Mol. Divers. 25, 2001–2015 (2021). https://doi.org/10.1007/s11030-020-10091-5
M.M. Li, C.S. Duan, Y.Q. Yu, D.Z. Xu, Dye. Pigment. 150, 202–206 (2018). https://doi.org/10.1016/j.dyepig.2017.12.007
H. Hassani, A. Nozarie, Asian J. Green Chem. 3, 59–69 (2018). https://doi.org/10.22631/ajgc.2017.101572.1032
A. Allahresani, B. Taheri, M.A. Nasseri, Res. Chem. Intermed. 44, 1173–1188 (2018). https://doi.org/10.1007/s11164-017-3160-8
A. Bazgir, G. Hosseini, R. Ghahremanzadeh, A.C.S. Comb, ACS Comb. Sci. 15, 530–534 (2013). https://doi.org/10.1021/co400057h
M. Rouhi, B. Sadeghi, M. Moslemin, Bulg. Chem. Commun. 50, 23–28 (2018)
S.G. Azarnier, M. Esmkhani, Z. Dolatkhah, S. Javanshir, Res. Sq. (2022). https://doi.org/10.1038/s41598-022-10102-5
S. Saneinezhad, L. Mohammadi, V. Zadsirjan, F.F. Bamoharram, M.M. Heravi, Sci. Rep. 10, 1–26 (2020). https://doi.org/10.1038/s41598-020-70738-z
B. Karmakar, A. Nayak, J. Banerji, Tetrahedron Lett. 53, 5004–5007 (2012). https://doi.org/10.1016/j.tetlet.2012.07.030
Y.K. Tailor, S. Khandelwal, K. Verma, R. Gopal, M. Kumar, ChemistrySelect 2, 5933–5941 (2017). https://doi.org/10.1002/slct.201700648
A. Rauof, M. Ali, Eng. Technol. Q. Rev. 3, 45–51 (2020). https://doi.org/10.5281/zenodo.3940869
N. Mohammadian, B. Akhlaghinia, Res. Chem. Intermed. 45, 4737–4756 (2019). https://doi.org/10.1007/s11164-019-03860-x
K. Hemmat, M.A. Nasseri, A. Allahresani, S. Ghiami, J. Organomet. Chem. 903, 120996 (2019). https://doi.org/10.1016/j.jorganchem.2019.120996
G. Shanthi, G. Subbulakshmi, P.T. Perumal, Tetrahedron 63, 2057–2063 (2017). https://doi.org/10.1016/j.tet.2006.12.042
M.A. Nasseri, B. Zakerinasab, Res. Chem. Intermed. 41, 5261–5270 (2015). https://doi.org/10.1007/s11164-014-1627-4
H.M. Meshram, D.A. Kumar, B.R.V. Prasad, P.R. Goud, Helv. Chim. Acta 93, 648–653 (2019). https://doi.org/10.1002/hlca.200900273
S.L. Zhu, S.J. Ji, Y. Zhang, Tetrahedron 63, 9365–9372 (2007). https://doi.org/10.1016/j.tet.2007.06.113
M. Dabiri, M. Bahramnejad, M. Baghbanzadeh, Tetrahedron 65, 9443–9447 (2009). https://doi.org/10.1016/j.tet.2009.08.070
A. Mobinikhaledi, N. Foroughifar, M.A.B. Fard, Synth. Commun. 41, 441–450 (2011). https://doi.org/10.1080/00397911003587507
A.R. Khorrami, P. Kiani, A. Bazgir, Monatshefte furr Chem. 142, 287–295 (2011). https://doi.org/10.1007/s00706-011-0446-1
Y. Li, H. Chen, C. Shi, D. Shi, S. Ji, J. Comb. Chem. 12, 231–237 (2010). https://doi.org/10.1021/cc9001185
D.R. Chandam, A.G. Mulik, D.R. Patil, M.B. Deshmukh, Res. Chem. Intermed. 42, 1411–1423 (2016). https://doi.org/10.1007/s11164-015-2093-3
A. Gharib, N.N. Pesyan, B.R.H. Khorasani, M. Roshani, J.W. Scheeren, Bulg. Chem. Commun. 45, 371–378 (2013)
J. Safaei-Ghomi, M. Tavazo, H. Shahbazi-Alavi, Zeitschrift fur Naturforsch.—Sect. B J. Chem. Sci. 74, 733–738 (2019). https://doi.org/10.1515/znb-2019-0091
A. Maleki, N. Ghamari, M. Kamalzare, RSC Adv. 4, 9416–9423 (2014). https://doi.org/10.1039/C3RA47366J
A. Maleki, M. Aghaei, N. Kamalzare, Int. J. Nanosci. Nanotechnol. 12, 215–222 (2016)
J. Safari, S.H. Banitaba, S.D. Khalili, Ultrason. Sonochem. 20, 401–407 (2013). https://doi.org/10.1016/j.ultsonch.2012.07.007
D. Nagargoje, P. Mandhane, S. Shingote, P. Badadhe, C. Gill, Ultrason. Sonochem. 19(1), 94–96 (2012). https://doi.org/10.1016/j.ultsonch.2011.05.009
J. Singh, M. Srivastava, J. Dutta, P.K. Dutta, Int. J. Biol. Macromol. 48, 170–176 (2011). https://doi.org/10.1016/j.ijbiomac.2010.10.016
G. Yin Li, Y. Ren Jiang, K. Long Huang, P. Ding, J. Chen, J. Alloys Compd. 466, 451–456 (2008). https://doi.org/10.1016/j.jallcom.2007.11.100
J.P. Chen, P.C. Yang, Y.H. Ma, T. Wu, Carbohydr. Polym. 84, 364–372 (2011). https://doi.org/10.1016/j.carbpol.2010.11.052
P. Saluja, K. Aggarwal, J.M. Khurana, Synth. Commun. 43, 3239–3246 (2013). https://doi.org/10.1080/00397911.2012.760130
M. Esmaeilpour, A.R. Sardarian, J. Javidi, Appl. Catal. A Gen. 445, 359–367 (2012). https://doi.org/10.1016/j.apcata.2012.09.010
G. Nabiyouni, M. Julaee, D. Ghanbari, P.C. Aliabadi, N. Safaie, J. Ind. Eng. Chem. 21, 599–603 (2015). https://doi.org/10.1016/j.jiec.2014.03.025
Acknowledgements
The authors acknowledge financial support from the research council of Shiraz University and are grateful for financial support from the Council of Iran National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mardaneh, P., Sardarian, A.R. Durable magnetite-chitosan core–shell nanoparticles as reusable green nanocatalyst for the benign one-pot three-component synthesis of spirooxindoles and spirochromenes at ambient temperature under both solvent-free and ultrasonic conditions in aqueous ethanol solution. J IRAN CHEM SOC 21, 211–225 (2024). https://doi.org/10.1007/s13738-023-02919-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02919-2