Abstract
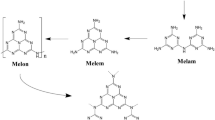
In this study, SiO2 nanoparticles were immobilized onto the surface of g-C3N4 nanosheets. The characterization of SiO2@g-C3N4 nanocomposite has been done by Fourier transform infrared spectra, X-ray diffraction, thermo-gravimetric analysis, scanning electron microscopy and transmission electron microscopy. The one-pot synthesis of spirooxindole derivatives was investigated via C–C bond formation reaction in the presence of a catalytic amount of SiO2@g-C3N4 nanocomposite using isatin (1 mmol), dimedone (1 mmol) and malononitrile (1 mmol) at ambient temperature in EtOH/water (1:1) media and the corresponding products were synthesized in excellent yields (90–95%) in short time (3–5 min). 4-Hydroxycoumarin was also subjected to the reaction instead of 1,3-diketones under similar conditions and the reactions proceeded in a longer time than for 1,3-diketones (8–11 min) with the products being obtained in 87–95% yields. The advantages of this catalyst include mild reaction conditions, a green and inexpensive nanocatalyst and green media. The nanocomposite was reused several times and applied in the next run without loss of reactivity.











Similar content being viewed by others
References
P.T. Anastas, M.M. Kirchhoff, Acc. Chem. Res. 35, 686 (2002)
L. Stanca, C. Sima, S.N. Petrache, A.I. Serban, A. Dinischiotu, Roman. Rep. Phys. 67, 1512 (2015)
I.A.M. Ibrahim, A.A.F. Zikry, M.A. Sharaf, J. Am. Sci. 6, 985 (2010)
L.L. Hench, J.K. West, Chem. Rev. 90, 33 (1990)
J. Zhang, J. Buffle, J. Colloid Interface Sci. 174, 500 (1995)
D. Huang, Z. Yang, X. Li, L. Zhang, J. Hu, Y. Su, N. Hu, G. Yin, D. He, Y. Zhang, Nanoscale 9, 109 (2017)
S. Kang, S.I. Hong, C.R. Choe, M. Park, S. Rim, J. Kim, Polymer 42, 879 (2001)
X. Miao, X. Shen, J. Wu, Z. Ji, J. Wang, L. Kong, M. Liu, C. Song, Appl. Catal. A Gen. 539, 104 (2017)
H. Liu, Z. Xu, Z. Zhang, D. Ao, Appl. Catal. A Gen. 518, 150 (2016)
L. Ge, C. Han, J. Liu, Y. Li, Appl. Catal. A Gen. 409–410, 215 (2011)
Q. Hao, X. Niu, C. Nie, S. Hao, W. Zou, J. Gea, D. Chen, W. Yao, Phys. Chem. Chem. Phys. 18, 31410 (2016)
B. Lin, C. Xue, X. Yan, G. Yang, G. Yang, Appl. Surf. Sci. 357, 346 (2015)
X. Wang, S. Wang, W. Hu, J. Cai, L. Zhang, L. Dong, L. Zhao, Y. He, Mater. Lett. 115, 53 (2014)
H.-B. Fang, Y. Luo, Y.-Z. Zheng, W. Ma, X. Tao, Ind. Eng. Chem. Res. 55, 4506 (2016)
S.C. Yan, Z.S. Li, Z.G. Zou, Langmuir 25, 10397 (2009)
X. Wang, K. Maeda, A. Thomas, K. Takanabe, Nat. Mater. 8, 76 (2009)
B. Long, J. Lin, X. Wang, J. Mater. Chem. A 2, 2942 (2014)
C. Conti, L.P. Monaco, N. Desideri, Bioorg. Med. Chem. 22, 1201 (2014)
A.L. Albiston, S. Diwakarla, R.N. Fernando, S.J. Mountford, H.R. Yeatman, B. Morgan, V. Pham, J.K. Holien, M.W. Parker, P.E. Thompson, S.Y. Chai, Br. J. Pharmacol. 164, 37 (2011)
N. Thomas, S. Zachariah, Asian J. Pharm. Clin. Res. 6, 11 (2013)
C.B. Sangani, N.M. Shah, M.P. Patel, R.G. Patel, J. Serb. Chem. Soc. 77, 1165 (2012)
Y.Y. Li, H. Chen, C. Shi, D. Shi, S. Ji, J. Comb. Chem. 12, 231 (2010)
C. Wu, R. Shen, J. Chen, C. Hu, Bull. Korean Chem. Soc. 34, 2431 (2013)
P. Rai, M. Srivastava, J. Singh, J. Singh, New J. Chem. 38, 3181 (2014)
R.Y. Guo, Z.M. An, L.P. Mo, S.-T. Yang, H.-X. Liu, S.-X. Wang, Z.-H. Zhang, Tetrahedron 69, 9931 (2013)
Y. Zou, Y. Hu, H. Liu, D. Shi, ACS Comb. Sci. 14, 38 (2012)
M.N. Elinson, A.S. Dorofeev, F.M. Miloserdov, G.I. Nikishin, Mol. Divers. 13, 47 (2009)
B.M. Rao, G.N. Reddy, T.V. Reddy, B.L.A. Prabhavathi Devi, R.B.N. Prasad, J.S. Yadav, B.V.S. Reddy, Tetrahedron Lett. 54, 2466 (2013)
M.A. Nasseri, A. Allahresani, A.A. Esmaeili, Lett. Org. Chem. 11, 91 (2014)
M.A. Nasseri, A. Allahresani, H. Raissi, Iran. J. Catal. 4, 33 (2014)
M.A. Nasseri, A. Allahresani, H. Raissi, RSC Adv. 4, 26087 (2014)
A. Allahresani, M.A. Nasseri, RSC Adv. 4, 60702 (2014)
A. Allahresani, M.A. Nasseri, A. Akbari, B.Z. Nasab, React. Kinet. Mech. Cat. 116, 249 (2015)
M.A. Nasseri, B. Zakerinasab, A. Allahresani, Iran. J. Catal. 5(2), 161 (2015)
M.A. Nasseri, F. Kamali, B. Zakerinasab, RSC Adv. 5, 26517 (2015)
A. Allahresani, M. A. Nasseri, A. Nakhaei, Res. Chem. Intermed. doi:10.1007/s11164-017-2994-4
S. Riyaz, A. Indrasena, A. Naidu, P.K. Dubey, Indian J. Chem. B 53, 1442 (2014)
S.P. Satasia, P.N. Kalaria, J.R. Avalani, D.K. Raval, Tetrahedron 70, 5763 (2014)
A. Hasaninejad, N. Golzar, M. Beyrati, A. Zare, M.M. Doroodmand, J. Mol. Catal. A Chem. 372, 137 (2013)
L.-M. Wang, N. Jiao, J. Qiu, J.-J. Yu, J.-Q. Liu, F.-L. Guo, Y. Liu, Tetrahedron 66, 339 (2010)
M.N. Elinson, R.F. Nasybullin, F.V. Ryzhkov, T.A. Zaimovskaya, G.I. Nikishi, Monatsh. Chem. 146, 631 (2015)
N. Azizi, S. Dezfooli, M.M. Hashemi, J. Mol. Liq. 194, 62 (2014)
T. He, Q.-Q. Zeng, D.-C. Yang, Y.-H. He, Z. Guan, RSC Adv. 5, 37843 (2015)
S.-L. Zhu, S.-J. Jia, Y. Zhang, Tetrahedron 63, 9365 (2007)
G.A. Gazieva, A.N. Izmest’ev, Chem. Heterocycl. Compd. 50, 1515 (2015)
Acknowledgements
The authors are grateful to the University of Birjand for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allahresani, A., Taheri, B. & Nasseri, M.A. A green synthesis of spirooxindole derivatives catalyzed by SiO2@g-C3N4 nanocomposite. Res Chem Intermed 44, 1173–1188 (2018). https://doi.org/10.1007/s11164-017-3160-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3160-8