Abstract
A novel series of quinoline-linked rhodanine bearing 1,2,3-triazole analogs (10a-l) have been designed and prepared. All the novel hybrids were analyzed and characterized by spectroscopic performances like 1H-NMR, 13C-NMR, and HR-MS analysis. The anticancer efficiency of final molecules was screened for their in vitro activity against the diverse cancer cells lines like HeLa (cervical carcinoma), MCF-7 (human breast), HT-29 (colon cancer), and Caco-2 (human epithelial). Amongst, compound (10c) exhibited more potent anticancer activity than Combretastatin-A4 as a standard drug against MCF7, Caco-2, HeLa, HT-29, and Caco-2 cancer cells with IC50 values of 3.67, 3.93, 4.92, and 6.83 μM, respectively. The overview of an electron-releasing substituent on the aryl ring exhibited potent anticancer activity. It is the first report to reveal the quinoline-linked rhodanine-bearing 1,2,3-triazole scaffolds as potential antitumor agents with inclusive docking analysis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant tumors and neoplasms are termed cancer. Cancer is the unrestrained, swift, and pathological production of abnormal cells that metastases to neighboring body parts and stands as one of the most intimidating afflictions in the world [1]. It is one of the foremost reasons for death. It results in about 9 million fatalities annually, which amounts to about 17% of total human casualties, and it is also the second leading cause of life loss next to heart illnesses [2]. By 2030, the threat of cancer is predicted to impact around 26 million new cases, causing 17 million deaths annually [3]. Chemotherapy is an essential strategy for cancer treatment. Thus, anticancer drugs are imperative for cancer therapy [4]. Although many therapeutic medications are approved, they are associated with drug resistance and toxicity problems [3,4,5]. Hence, to alleviate these problems, there remains an imperative necessity to develop the most potential and innocuous chemotherapeutic agents with lesser side effects and improved efficacy.
Nitrogen-containing heterocyclic compounds play a crucial role in numerous biological processes and pharmaceutical applications due to their diverse chemical properties [6, 7]. At least one nitrogen atom, which confers special reactivity and functionality, is what distinguishes these compounds from other ones. Numerous strategies, such as conventional organic synthesis methods and cutting-edge catalytic technologies, can be used to create heterocyclic molecules that include nitrogen. The modification of easily accessible starting elements is a common step in their synthesis in order to create intricate ring structures. These substances have also attracted a lot of interest as enzyme inhibitors, showing the capacity to modify enzymatic activity and control biochemical processes. Nitrogen-containing heterocyclic compounds have a tremendous promise for drug development and the treatment of many illnesses, including cancer, infectious diseases, and neurological disorders, by specifically targeting particular enzymes. Their capacity to suppress enzyme activity offers a useful method for creating treatments with improved selectivity and effectiveness [8,9,10,11,12].
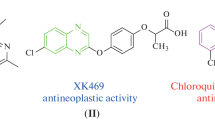
Heterocyclic compounds are versatile and promising synthons in drug discovery [13]. Triazole-based heterocycles are particularly important essential pharmacophores [14]. A robust metabolic profile labels 1,2,3-triazoles, which engross these heterocyclic compounds in drug design as linker moieties owing to their stability toward metabolic degeneration under physiological conditions under a broad extent of pH and redox environments [15]. They engage in non-covalent interactions like H-bonds, dipole–dipole bonds, hydrophobic, and Vander Waals interactions with bio-molecular targets like DNA [16]. Consequently, they can alter biological activities and are prevalent as pharmacophores in many exciting drugs [17]. Further, these moieties are excellent chemical scaffolds of low toxicity and diverse pharmacological activity, including anti-proliferative, anti-retroviral, anti-convulsant, anti-inflammatory, and antimicrobial [16,17,18,19]. 1,2,3-Triazoles display anticancer action toward leukemia, breast, prostate, human hepatoma, lung, cervical, colon carcinoma, gastric, ovarian, and other malignancies [20]. The arrival of bioactive molecules having 1,2,3-triazole basic comprising antibiotic (Cefatrizine), anticancer carboxyamidotriazole (CAI), anti-bacterial (Tazobactum), has led the approach for the improvement of 1,2,3-triazole having molecules (Fig. 1).
Rhodanine or 2-thioxothiazolidin-4-one (TZs) scaffold is an important constituent of many heterocyclic compounds that feature in several pharmaceutical targets and natural products of medicinal interest (Fig. 2). TZs are organic heterocycles comprising a five-membered saturated ring with a thioether and an amine group at positions 1 and 3 [21, 22]. It is a sulfur analog of oxazolidine. Functionalized TZs are reported to be a novel heterocyclic scaffold of medicinal importance and deliver wide-ranging biological behaviors like antidiabetic, antioxidant, antimicrobial, anti-tubercular, anti-convulsant, anti-inflammatory, and cytotoxic activities [23,24,25,26]. Quinoline belonging to the benzo-pyridine family, represents a vital class of N-heterocycles and is widely distributed in natural products, drugs, and functional materials [27]. Quinoline finds application in areas like dye manufacturing, solvent for resins and terpenes, and in the production of pesticide precursors and other specialty chemicals [27,28,29]. Quinoline alkaloids exhibit numerous biological activities, including antimicrobial, anticancer, anti-inflammatory, and antimalarial. 2-Pyridyl-quinoline is an established biomarker for neuro-inflammation [30,31,32,33]. Additionally, quinoline derivatives display inhibition towards EGFR-TK and antipsychotic actions and are ubiquitous in drug research [34, 35].
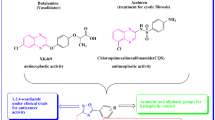
In our continuous investigations, several heterocyclic molecules were synthesized and screened for their biological activity [36,37,38,39,40,41,42]. Recently, we have reported that novel dinitrophenylpyrazole bearing 1,2,3-triazole exhibited higher cytotoxicity than Combretastatin-A4 as a reference drug [43]. Similarly, thieno-[2,3-d]-pyrimidine tethered 1,2,3-triazole analogs showed more potent antioxidant activity than ascorbic acid [44]. Hybrid drug design has evolved as a primary tool towards advances in anticancer therapies wherein two or more pharmacophores are combined to realize synergistic effects culminating in new and better modes of action. As both thiazolidine and quinoline moieties have due to recognition as potent pharmacological units, we strategized to incorporate both attractive groups in a single molecule to verify their anticipated anti-proliferative activities. Hence, in this investigation, we aimed to describe the amalgamation of chloroquinoline moiety, rhodanine, and 1,2,3-triazole scaffold structure and evaluate the in vitro cytotoxicity and molecular docking study alongside a board of cancer cell lines.
Experimental section
Chemistry
General preparation of 6-chloro-2-methylquinoline (3) [45]
A solution of crotonaldehyde (2, 1 mmol) in toluene (5 mL), and 4-chloroaniline (1, 0.5 mmol) in aqueous hydrochloric acid (HCl) (2 mL) was taken in a reaction flask and stirred for 12 h at 100 °C. As examined by thin layer chromatography (TLC), the reaction mass was cooled, and followed by the separation of toluene. Now to the aqueous layer, ethyl acetate (5 mL) the solution was added. Then, it was separated, dried using anhydrous sodium sulfate (Na2SO4), and concentrated. Further, it was subjected to purification by column chromatographic method to afford product 3 (63%).
General synthesis of 6-chloroquinoline-2-carbaldehyde (4) [45]
The mixture of compound 3 (1 mmol) in 1,4-dioxane (8 mL) and selenium dioxide (SeO2, 1.2 mmol) in a reaction flask was stirred and refluxed for 15 min at 100 °C. As observed by TLC, after the accomplishment of the reaction, the compound was extracted by 5 mL of EtOAc solvent, dried with Na2SO4, and concentrated under reduced pressure to achieve a crude compound. Further, the crude was purified by the column chromatographic technique to obtain a pure compound (4) using an eluent (hexane: ethyl acetate) in the ratio of 9:1.
General approach for the preparation of quinoline-rhodanine derivative (6) [46]
A combination of compound 4 (1 mmol), rhodanine 5 (1.1 mmol), sodium acetate (CH3COONa, 3 mmol), and acetic acid (15 mL) was taken into round bottom flask and heated under reflux for 3 h. TLC detected the completion of the reaction mass. Later, the reaction mass was cooled to r.t and poured into crushed ice to obtain solid. Then it was filtered, washed, and dried under reduced vacuum to afford crude product. Further, pure compound (6) with excellent yield was formed by recrystallization with ethanol (EtOH) solvent.
General synthesis of molecule (8) [45]
To a suspension of the above compound (6) (1 mmol) in dry DMF (5 mL), propargyl bromide (7) (2.1 mmol), and potassium carbonate (K2CO3, 2.5 mmol) were added to a reaction flask and stirred at r.t for 2 h. The reaction progress was checked by TLC, then the reaction mass was transferred into a crushed ice flask to obtain a solid. Later, the solid was filtered, washed, and dried under a reduced pressure to afford the pure product (8) followed by recrystallization with EtOH medium.
General synthesis of final molecules (10a-l)
To a solution of 8 (1 mmol) in aqueous dichloromethane (H2O:DCM = 1:1, 10 mL), copper sulphate (0.05 mmol), and sodium ascorbate (C6H7O6Na, 0.13 mmol) were taken into the reaction vessel and stirred at r.t for a few minutes. Later, suitably substituted azides (9a-l) (2 mmol) were added and were continued to be stirred for 2 h under the same conditions, after checking by TLC, the reaction was poured into ice-cold water to obtain a solid. Then, the solid was filtered, washed, and dried under a reduced vacuum. Further, the crude was purified by column chromatography to afford the target molecules (10a-l) in excellent yields.
(Z)-5-((6-chloroquinolin-2-yl)methylene)-3-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl) methyl)-2-thioxothiazolidin-4-one (10a)
Yellow colour solid; Yield: 81%; 1H NMR (400 MHz, DMSO-d6) δ 9.08 (s, 1H, triazole-CH), 8.85 (s, 1H, CH), 8.14 (dd, J = 8.0, 1.3 Hz, 1H, Ar–H), 8.07 (t, J = 2.0 Hz, 1H, Ar–H), 7.94 (d, J = 8.9 Hz, 2H, Ar–H), 7.82 (dd, J = 15.3, 1.5 Hz, 1H, Ar–H), 7.58 (d, J = 8.1 Hz, 1H, Ar–H), 7.52 (dd, J = 15.1, 1.0 Hz, 1H, Ar–H), 7.28 (d, J = 8.8 Hz, 2H, Ar–H), 5.38 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 168.85, 161.48, 157.45, 153.41, 146.30, 143.54, 137.54, 134.19, 131.63, 130.75, 128.61, 126.70, 126.54, 125.26, 123.26, 120.96, 119.98, 118.77, 115.38, 61.23; HRMS of [C22H13N6O3S2Cl + H]+ (m/z) 509.0473; Calcd: 509.0488.
(Z)-5-((6-Chloroquinolin-2-yl)methylene)-3-((1-(3-nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl)-2-thioxothiazolidin-4-one (10b)
Yellow colour solid; Yield: 83%; 1H NMR (400 MHz, DMSO-d6) δ 9.09 (s, 1H, triazole-CH), 8.85 (s, 1H, CH), 8.28 (s, 1H, Ar–H), 8.14 (d, J = 7.7 Hz, 1H, Ar–H), 8.01–7.91 (m, 3H, Ar–H), 7.89 (d, J = 8.8 Hz, 1H, Ar–H), 7.81 (dd, J = 11.1, 4.2 Hz, 1H, Ar–H), 7.65 (d, J = 8.1 Hz, 1H, Ar–H), 7.52 (t, J = 7.5 Hz, 1H, Ar–H), 5.38 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 168.02, 163.76, 161.33, 156.46, 151.71, 145.55, 142.43, 135.23, 130.55, 130.47, 130.40, 130.28, 130.20, 128.02, 127.88, 127.77, 125.03, 121.28, 119.18, 115.93, 115.72, 61.88; HRMS of [C22H13N6O3S2Cl + H]+ (m/z) 509.1989; Calcd: 509.1979.
(Z)-5-((6-Chloroquinolin-2-yl)methylene)-3-((1-(2-nitrophenyl)-1H-1,2,3-triazol-4-yl) methyl)-2-thioxothiazolidin-4-one (10c)
White colour solid; Yield: 85%; 1H NMR (400 MHz, DMSO-d6) δ 9.25 (s, 1H, triazole-CH), 8.86 (s, 1H, CH), 8.76 (t, J = 2.1 Hz, 1H, Ar–H), 8.43 (d, J = 8.1 Hz, 1H, Ar–H), 8.14 (d, J = 6.7 Hz, 1H, Ar–H), 7.93 (dd, J = 16.7, 8.5 Hz, 3H, Ar–H), 7.82 (t, J = 6.9 Hz, 1H, Ar–H), 7.65 (d, J = 7.9 Hz, 1H, Ar–H), 7.52 (dd, J = 15.1, 1.0 Hz, 1H, Ar–H), 5.41 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 168.83, 161.47, 157.45, 153.40, 148.52, 146.30, 143.82, 137.09, 134.24, 131.56, 130.75, 126.71, 126.54, 126.23, 126.20, 125.29, 123.55, 123.23, 120.97, 115.39, 114.91, 61.21; HRMS of [C22H13N6O3S2Cl + H]+ (m/z) 509.2272; Calcd: 509.2266.
(Z)-5-((6-Chloroquinolin-2-yl)methylene)-2-thioxo-3-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl) methyl)thiazolidin-4-one (10d)
Light yellow colour solid; Yield: 86%; 1H NMR (400 MHz, CDCl3) δ 8.81 (s, 1H, triazole-CH), 8.21 (dd, J = 8.0, 1.1 Hz, 1H, Ar–H), 7.99 (s, 1H, CH), 7.80 (d, J = 8.8 Hz, 2H, Ar–H), 7.67 (d, J = 15.2 Hz, 1H, Ar–H), 7.60 (d, J = 7.6 Hz, 1H, Ar–H), 7.39 (t, J = 7.1 Hz, 1H, Ar–H), 7.25 (t, J = 8.6 Hz, 1H, Ar–H), 7.05 (d, J = 8.7 Hz, 2H, Ar–H), 5.31 (s, 2H, CH2), 2.58 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 166.24, 161.53, 158.75, 156.86, 154.08, 146.45, 134.28, 130.86, 127.20, 126.84, 126.39, 126.06, 123.18, 122.80, 121.48, 120.42, 117.86, 117.63, 115.21, 61.99, 29.70; HRMS of [C23H16N5OS2Cl + H]+ (m/z) 478.1547; Calcd: 478.1543.
(Z)-5-((6-Chloroquinolin-2-yl)methylene)-2-thioxo-3-((1-(m-tolyl)-1H-1,2,3-triazol-4-yl) methyl)thiazolidin-4-one (10e)
Brown colour solid; Yield: 72%; 1H NMR (400 MHz, CDCl3) δ 8.82 (s, 1H, triazole-CH), 8.22 (d, J = 7.5 Hz, 1H, Ar–H), 8.00 (s, 1H, CH), 7.81 (d, J = 8.8 Hz, 2H, Ar–H), 7.64 (d, J = 8.8 Hz, 2H, Ar–H), 7.46 (d, J = 8.6 Hz, 2H, Ar–H), 7.40 (t, J = 7.9 Hz, 1H, Ar–H), 7.07 (d, J = 8.8 Hz, 2H, Ar–H), 5.32 (s, 2H, CH2), 2.60 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 166.66, 162.62, 156.39, 150.37, 147.53, 145.92, 142.62, 137.70, 135.13, 131.20, 130.30, 130.20, 130.16, 129.76, 129.63, 129.18, 128.15, 125.54, 123.99, 121.32, 119.70, 58.65, 23.38; HRMS of [C23H16N5OS2Cl + H]+ (m/z) 478.0482; Calcd: 478.0490.
(Z)-5-((6-Chloroquinolin-2-yl)methylene)-2-thioxo-3-((1-(o-tolyl)-1H-1,2,3-triazol-4-yl) methyl)thiazolidin-4-one (10f)
Yellow colour solid; Yield: 82%; 1H NMR (400 MHz, CDCl3) δ 8.89 (s, 1H, triazole-CH), 8.12 (d, J = 8.1 Hz, 1H, Ar–H), 7.97 (s, 1H, CH), 7.88 (d, J = 8.7 Hz, 2H, Ar–H), 7.82 (dd, J = 8.2, 7.1 Hz, 1H, Ar–H), 7.74 (dd, J = 7.3, 5.0 Hz, 2H, Ar–H), 7.66 (dd, J = 6.9, 5.8 Hz, 2H, Ar–H), 7.46 (t, J = 7.4 Hz, 1H, Ar–H), 5.41 (s, 2H, CH2), 2.65 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 166.24, 161.59, 158.76, 154.09, 146.55, 144.46, 144.18, 134.23, 133.93, 131.02, 130.85, 130.16, 128.10, 127.21, 126.89, 126.34, 126.05, 125.69, 124.59, 121.53, 115.27, 61.99, 30.93; HRMS of [C23H16N5OS2Cl + H]+ (m/z) 478.8751; Calcd: 478.8768.
(Z)-3-((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-5-((6-chloroquinolin-2-yl) methylene)-2-thioxothiazolidin-4-one (10g)
Yellow colour solid; Yield: 84%; 1H NMR (400 MHz, DMSO-d6) δ 9.22 (s, 1H, triazole-CH), 8.86 (s, 1H, CH), 8.47 (d, J = 9.1 Hz, 2H, Ar–H), 8.26 (d, J = 9.1 Hz, 2H, Ar–H), 8.14 (d, J = 6.8 Hz, 1H, Ar–H), 7.95 (d, J = 8.8 Hz, 1H, Ar–H), 7.82 (t, J = 6.9 Hz, 1H, Ar–H), 7.65 (d, J = 8.0 Hz, 1H, Ar–H), 7.52 (t, J = 7.6 Hz, 1H, Ar–H), 5.41 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 168.82, 161.45, 157.45, 153.40, 146.80, 146.30, 144.03, 140.74, 134.24, 130.75, 126.71, 126.54, 126.24, 125.56, 125.31, 123.52, 120.97, 120.74, 115.38, 61.17; HRMS of [C22H13N5OS2Cl2 + H]+ (m/z) 497.0927; Calcd: 497.0936.
(Z)-3-((1-(3-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-5-((6-chloroquinolin-2-yl) methylene)-2-thioxothiazolidin-4-one (10h)
Brown colour solid; Yield: 71%; 1H NMR (400 MHz, DMSO-d6) δ 9.25 (s, 1H, triazole-CH), 8.86 (s, 1H, CH), 8.76 (s, 1H, Ar–H), 8.43 (d, J = 7.9 Hz, 1H, Ar–H), 8.34 (d, J = 7.0 Hz, 1H, Ar–H), 8.14 (d, J = 7.6 Hz, 1H, Ar–H), 7.95–7.88 (m, 2H, Ar–H), 7.82 (t, J = 7.4 Hz, 1H, Ar–H), 7.65 (d, J = 8.1 Hz, 1H, Ar–H), 7.52 (t, J = 7.5 Hz, 1H, Ar–H), 5.41 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 168.82, 161.47, 157.45, 153.40, 148.52, 146.31, 143.82, 137.09, 134.23, 131.56, 130.75, 126.71, 126.54, 126.23, 126.20, 125.30, 123.56, 123.23, 120.97, 115.39, 114.91, 61.22; HRMS of [C22H13N5OS2Cl2 + H]+ (m/z) 497.0947; Calcd: 497.0936.
(Z)-3-((1-(2-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-5-((6-chloroquinolin-2-yl) methylene)-2-thioxothiazolidin-4-one (10i)
Brown colour solid; Yield: 75%; 1H NMR (400 MHz, DMSO-d6) δ 9.01 (s, 1H, triazole-CH), 8.85 (s, 1H, CH, Ar–H), 8.15 (d, J = 7.7 Hz, 1H, Ar–H), 7.96 (dd, J = 12.8, 8.8 Hz, 3H, Ar–H), 7.81 (d, J = 7.2 Hz, 1H, Ar–H), 7.75 (d, J = 8.6 Hz, 1H, Ar–H), 7.66 (d, J = 8.0 Hz, 1H, Ar–H), 7.52 (t, J = 7.7 Hz, 1H, Ar–H), 7.28 (d, J = 8.6 Hz, 1H, Ar–H), 5.37 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 167.32, 158.58, 156.54, 153.04, 145.23, 142.27, 135.37, 131.24, 131.16, 129.87, 129.49, 129.41, 129.33, 127.90, 127.18, 127.16, 124.70, 121.77, 121.24, 118.96, 115.63, 61.42; HRMS of [C22H13N5OS2Cl2 + H]+ (m/z) 497.0926; Calcd: 497.0936.
(Z)-3-((1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)-5-((6-chloroquinolin-2-yl) methylene)-2-thioxothiazolidin-4-one (10j)
Yellow colour solid; Yield: 75%; 1H NMR (400 MHz, DMSO-d6) δ 8.89 (s, 1H, triazole-CH), 8.85 (s, 1H, CH), 8.24 (dd, J = 8.1, 1.0 Hz, 1H, Ar–H), 8.14 (dd, J = 7.9, 1.0 Hz, 1H, Ar–H), 7.95 (d, J = 8.7 Hz, 2H, Ar–H), 7.84–7.79 (m, 1H, Ar–H), 7.65 (d, J = 8.1 Hz, 1H, Ar–H), 7.52 (t, J = 7.5 Hz, 1H, Ar–H), 7.29 (d, J = 8.8 Hz, 2H, Ar–H), 5.40 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d6) δ 168.83, 161.53, 157.46, 153.42, 146.29, 144.03, 143.01, 134.44, 131.30, 130.74, 129.00, 127.65, 126.70, 126.54, 126.23, 125.54, 125.26, 120.96, 115.35, 61.09; HRMS of [C22H13N5OS2ClBr + H]+ (m/z) 541.0753; Calcd: 541.0742.
(Z)-3-((1-(2-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)-5-((6-chloroquinolin-2-yl)methylene)-2-thioxothiazolidin-4-one (10k)
Yellow colour solid; Yield: 77%; 1H NMR (400 MHz, CDCl3) δ 8.89 (s, 1H, triazole-CH), 8.29 (d, J = 6.8 Hz, 1H, Ar–H), 8.05 (s, 1H, CH), 7.88 (d, J = 8.8 Hz, 2H, Ar–H), 7.74 (t, J = 6.9 Hz, 1H, Ar–H), 7.67 (d, J = 7.8 Hz, 1H, Ar–H), 7.62 (d, J = 8.5 Hz, 2H, Ar–H), 7.46 (dd, J = 11.0, 4.0 Hz, 1H, Ar–H), 7.33 (d, J = 8.1 Hz, 1H, Ar–H), 5.38 (s, 2H, CH2); 13C NMR (100 MHz, CDCl3) δ 168.30, 165.54, 161.72, 156.45, 151.50, 145.63, 142.46, 135.20, 131.90, 131.83, 130.57, 130.51, 130.49, 130.42, 130.10, 130.02, 128.07, 125.14, 122.56, 121.31, 119.26, 61.83; HRMS of [C22H13N5OS2ClBr + H]+ (m/z) 541.0753; Calcd: 541.0742.
(Z)-5-((6-Chloroquinolin-2-yl)methylene)-3-((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)-2-thioxothiazolidin-4-one (10l)
Light yellow colour solid; Yield: 64%; 1H NMR (400 MHz, DMSO-d6) δ 8.89 (s, 1H, triazole-CH), 8.85 (s, 1H, CH), 8.15 (d, J = 6.7 Hz, 1H, Ar–H), 7.93 (d, J = 8.8 Hz, 2H, Ar–H), 7.82 (d, J = 9.0 Hz, 1H, Ar–H), 7.65 (d, J = 8.0 Hz, 1H, Ar–H), 7.57–7.47 (m, 1H, Ar–H), 7.28 (d, J = 8.8 Hz, 1H, Ar–H), 7.18 (d, J = 8.8 Hz, 2H, Ar–H), 5.36 (s, 2H, CH2), 3.84 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6) δ 168.82, 160.71, 157.44, 153.41, 146.30, 143.09, 134.25, 130.75, 130.61, 126.71, 126.55, 126.25, 125.47, 123.06, 121.85, 120.97, 115.45, 115.37, 114.88, 61.21, 55.71; HRMS of [C23H16N5O2S2Cl + H]+ (m/z) 494.1256; Calcd: 494.1247.
Anticancer activity analysis
Cell culture
The prepared target molecules were solubilized in dimethyl sulfoxide (DMSO) and the final concentration of DMSO was maintained to lower than 0.1% (v/v). A 0.45 μm syringe filter was used to filter the solutions and stored at –20 °C for in vitro screening. The cell lines (HeLa, MCF-7, Caco-2, and HT-29) were grown in an EMEM media augmented with heat-inactivated bovine serum (10% FBS), L-glutamine (1%,) and penicillin or streptomycin (1%), and cultivated at 37 °C in CO2 (5%). All experiments were performed in at least two replicates.
Cytotoxicity procedure
Cytotoxicity evaluation of the synthetic molecules against all cell lines was measured using the MTT reduction process via in vitro method [47]. The breast adenocarcinoma (MCF-7), cervical carcinoma (HeLa), HT-29 (colon cancer), and human epithelial colorectal adenocarcinoma (Caco-2) cell lines were discretely trypsinized and plated in 96-multiwell plates (density 2.1–2.5 × 104 cells/well) and left overnight at 37° for incubation in 0.1 mL complete growth medium to attain 80% confluence. Next, the cells were exposed to different concentrations (25–200 µM in DMSO) of target molecules, followed by another 48 h incubation. After incubation is complete, the medium was removed from the wells, 0.1 mL of fresh medium, and MTT reagent (5 mg/mL in sterile PBS at pH 7.4) was added and incubated for 4 h. The medium was removed again, and wells were replaced with DMSO (0.1 mL). Absorbance readings were noted at 570 nm with a Vacutec MR-96A microplate reader. All the results were equated to the cytotoxicity properties of Combretastatin-A4 as a standard drug [48, 49].
Molecular docking analysis
Swiss Dock, an online server was used to studying molecular docking. DeltaG, full fitness score hydrogen binding energy and interactions were the factors considered to evaluate molecular docking studies using UCSF chimera software. Primary structural construction and 3D innovation were made possible using ChemDraw and molinspiration tools respectively. The Brain Or IntestinaL EstimateD permeation method or commonly termed as BOILED-EGG model is used to evaluate gastrointestinal and brain penetration absorption. Facilitated by UCSF chimera/other software, we docked all our tetrazole derivatives—the new drug generations—with their respective anticancer targets (PDB:1W98).
Results and discussion
Chemistry
The designated molecular scaffold synthesis is illustrated in Scheme 1. The 6-chloro-2-methyl quinoline (3) was synthesized via condensation of 4-chloroaniline with crotonaldehyde. Next, the molecule (3) on reaction with SeO2 and 1,4-dioxane through oxidation obtain 6-chloroquinoline-2-carbaldehyde as compound (4). Then, molecule (4) on condensation with rhodanine (5) in CH3COOH under reflux conditions to yield 5-((6-chloroquinolin-2-yl) methylene)-2-thioxothiazolidin-4-one (6). Later the formed molecule (6) was N-alkylated by propargyl bromide under basic conditions at room temperature, affording the corresponding propargylated compound (8). Later, compound (8) was cyclized with different aryl azides to yield the desired chloroquinoline bearing 1,2,3-triazole hybrids (10a-l). These novel synthesized analogs were established and analyzed by spectral studies (1H NMR, 13C NMR, and HR-MS). The The 1H NMR spectra of compound 10a reveal that three singlet protons were confirmed at δ 9.08 ppm for 1,2,3-triazole, δ 8.85 ppm for imine, and 5.38 ppm for the CH2 group. The existence of various multiplets signals at δ value 7.28–8.14 ppm supports the existence of aromatic protons. Additionally, the structures of the target hybrids were confirmed by their carbon-NMR (13C NMR). In the 13C NMR spectrum, carbonyl carbon and thiocarbon of rhodanine skeleton signal is shown the range at δ 158.58–165.54 ppm, and 166.24–168.85 ppm and N-CH2 in between rhodanine and triazole moiety of methylene carbon appeared at the range of 58.65–61.99 ppm. Moreover, the molecular ion peak was detected at m/z 509.0473 (M + H)+ in the HRMS spectrum. All the synthesized target molecules were analyzed and proved by the spectral analysis (1H NMR, 13C NMR, and HR-MS) and their spectra were given in the supplementary materials (SIII).
Biological screening
The most prominent models used in cytotoxic studies and drug discovery are the cell lines of Tumor cells which are crucial in therapeutic and mechanical comprehension as preclinical modeling systems. Breast tumor cells, lung, and colon cell lines are the most popularly studied tumors in human tumor cell lines. In the present study, HeLa (cervical carcinoma), HT-29 (colon cancer), Caco-2 (human epithelial colorectal adenocarcinoma), and MCF-7 (human breast adenocarcinoma), cell lines were chosen to study the in vitro cytotoxic response toward the newly synthesized compounds (10a-1). Combretastatin-A4 was utilized as the reference drug.
Table 1 displays the IC50 values obtained for the tested molecules. The newly fabricated compounds showed excellent cytotoxic activity towards the chosen tumor cell lines as suggested by the mean IC50 values. Compound 10c exhibited considerable antitumor activity against the cancer cell lines (HeLa, HT-29, MCF-7, and Caco2) with IC50 values of (4.92, 6.83, 3.67, and 3.93 μM), respectively. Compounds 10k and 10a also revealed eminent anticancer activity against the series of the chosen cell lines, with the values range (IC50: 3.72–9.12 μM).
All structural scaffolds, like the triazole moiety, rhodanine moiety, quinoline moiety, and different substituted phenyl components showed comparable anticancer action. However, depending on the electron-withdrawing substitutes and capricious positions in the phenyl group, the target compounds exhibited diverse behavior owing to a limited structural activity relationship. The electron-donating group on the phenyl ring of compounds (Me and OMe) showed minimal cytotoxic effect amidst the tested molecules, as observed from its IC50 values. Compounds (10d, 10e, 10l, and 10f) exhibited a clear amplification in potency compared to the electron-donating functional compounds (10d, 10e, 10l, and 10f) due to the introduction of an electron-withdrawing substituent on the aromatic ring. The most active derivative among the synthesized molecules was 10c bearing the 2-nitro group on the phenyl ring, whereas compounds 10b and 10a were next in line bearing 3-nitro and 4-nitro groups on the phenyl ring. Compounds 10c, 10b, and 10a were more cytotoxic amid the whole series of eth synthesized compounds. The substitution groups on the phenyl ring showed cytotoxicity in the following (2-NO2 > 3-NO2 > 4-NO2 > 2-Cl > 2-Br > 3-Cl > 4-Cl > 4-Br). Lipophilic and electronegative substituents on the phenyl ring impart cytotoxic potency against cancer cell lines. The halo and nitro substitutions on the phenyl ring are known to perform a distinctive role in medicinal chemistry in terms of inducing anti-tumor properties to the prepared compounds. Therefore, it might be summarized that the phenyl rings with –NO2, –Cl, and –Br groups (strong electron withdrawing groups) in ambient positions influence their potent activity.
Molecular docking screening
The drug designing and development is very time taking and expensive process. Computational studies are very cost effective and less time-consuming. These studies/tools can easily screen huge number of compounds before going for in-vitro/ in-vivo process. Evaluation of physicochemical and pharmacokinetic properties have been done before molecular docking simulations. Physicochemical and pharmacokinetic properties have been evaluated with the help of an online server SwissADME and pkCSM tool respectively [50]. The analysis of physicochemical properties is done with the help of Lipinski rule (RO5 rule), which is based on certain parameters like MW (molecular weight), TPSA (topological surface area), HBA (hydrogen bonding acceptors), HBD (hydrogen bonding donors), lipophilicity, logP, nRot (number of rotational bonds), Violations etc. According to Lipinski rule of five (RO5 rule), MW < 500 Dalton, TPSA (TPSA) ≤ 140 Å2, HBD < 5, HBA < 10, Violations < 4 for good bioavailability of drug. It is observed that all derivatives followed Lipinski’s rule of five (RO5 rule) they follow range of MW < 500 Dalton, TPSA (TPSA) ≤ 140 Å2, HBD < 5, HBA < 10, Violations < 4. Derivatives 10a, 10j, 10c, 10b, and 10k showed only one violation as their MW is more than 500 which is against the RO5 rule (Table 2). Gastrointestinal absorption and brain penetration effect is one of the important physicochemical parameters which has been analysed with the help of ‘BOILED-EGG method (Fig. 3). The preliminary structural innovation was carried out using the ChemDraw tool whereas the molinspiration tool, (online server) was used for designing the 3D structures, (Table S1). According to this model if any derivatives fall under white region then it could show good gastrointestinal absorption but if derivatives fall under yellow region the compound could show good brain penetration effect. Here, all screened derivatives showed good gastrointestinal absorption as they fall under the white region of egg. The online server pkCSM tool was used to evaluate the Pharmacokinetic characteristics which is based on ADMET (absorption, distribution, metabolism, excretion, toxicity). These parameters are used to analyse the toxicity, absorption, distribution etc. of the compounds. It has been observed that all screened derivatives showed zero skin sensitization and no AMES as well as renal toxicity. All derivatives showed good drug bioavailability as they follow the range of ADMET (Tables 3 and 4). Molecular docking studies has been carried out after analysing physicochemical and pharmacokinetic properties. SwissDock, an online server was employed for Molecular docking studies. Molecular docking based on “Lock and Key hypothesis” where derivative acts as lock whereas protein/target acts key. Active poses have to be analysed where derivative could show hydrogen binding interactions with protein. All derivatives have been cleaned in “CLEAN 2D & CLEAN 3D” with the help of MarvinSketch tool by removing all water molecules and hetero atoms. Protein data bank, PDB ID is retrieved from rcsb.org. The key parameters of molecular docking studies are hydrogen binding energy modes, DeltaG, hydrogen binding interactions, full fitness score, etc., which were evaluated utilizing UCSF chimera software. Hydrogen binding interactions should be high for analysing active poses of protein/target with the derivatives/compounds. There should be high negative value of full fitness score and greater negative DeltaG value for more feasibility. With the help of UCSF chimera software, we docked all our screened derivatives—the new drug generation's—with their respective anticancer target (PDB:1W98). PDB ID: 1W98 is the structural basis of CDK2 activation by cyclin E. Cyclin E acts as an activator pf posho-CDK2. This is majorly expressed in cancerous cells. In metazoans, it plays key role in cell cycle progression and helps in entry to the cell cycle from G0 quiescent phase. PDB: 1W98, showed good results with heterocyclic compounds that’s why we have done the molecular docking of our heterocyclic compounds first before going in-vitro/in vivo as computational tools/in-silico tools/molecular docking are less time consuming, cost effective and help in screening of best bioactive compounds against respective targets. All compounds showed very good results owing to satisfactory binding energy values (DeltaG), Full fitness Score, and hydrogen binding interactions. 10b, 10i, and 10l derivatives showed the best results based on the highest DeltaG energy (− 8.21), (Table 5). Hydrogen binding interactions of derivatives with ribbons of protein/target are clearly visible in docked images (Fig. 4).
Conclusion
A series of twelve novel chloroquinoline-1,2,3-triazole-rhodanine hybrids (10a-l) were synthesized and evaluated for anticancer activity and subjected to physicochemical characteristics. The anticancer activity was screened against the human cell lines (HT-29, MCF-7, Caco-2, and HeLa) via the MTT methodology. Among all the tested hybrids, compounds bearing the ortho-nitro group substituted in the phenyl ring showed promising anticancer activity (IC50 = 3.67 μM) against the human breast cancer cell line (MCF-7), compared to the standard drug Combretastatin-A4 (IC50 = 3.72 μM). Furthermore, molecular docking analysis was established, and these active compounds showed effective bonding interactions. Thus, these reported hybrids are ideal candidates to be further development of anticancer agents.
References
H. Wang, Q. Meng, J. Qian, M. Li, C. Gu, Y. Yang, Review: RNA-based diagnostic markers discovery and therapeutic targets development in cancer. Pharmacol. Ther. 234, 108123 (2022)
A.J. Kim, D.S. Hong, G.C. George, Dietary influences on symptomatic and non-symptomatic toxicities during cancer treatment: A narrative review. Cancer Treat. Rev. 108, 102408 (2022)
F. Bray, B. Moller, Predicting the future burden of cancer. Natural Rev. Cancer. 6, 63–74 (2006)
B. Magalhaes, C. Fernandes, J.M. Martinez-Galiano, L. Lima, C. Santos, Cancer patients’ experiences on self-management of chemotherapy treatment-related symptoms: A systematic review and thematic synthesis. Eur. J. Oncol. Nurs. 49, 101837 (2020)
M. Sharma, A.K. Bakshi, N. Mittapelly, S. Gautam, D. Marwaha, N. Rai, N. Singh, P. Tiwari, N. Agarwal, A. Kumar, P.R. Mishra, Recent updates on innovative approaches to overcome drug resistance for better outcomes in cancer. J. Control. Release 346, 43–70 (2022)
S. Gupta, M. Lakshman, Magnetic nano cobalt ferrite: An efficient recoverable catalyst for synthesis of 2,4,5-trisubstituted imidazoles. J. Med. Chem. Sci. 2, 51–54 (2019)
M. Khoobi, A. Foroumadi, S. Emami, M. Safavi, G. Dehghan, B.H. Alizadeh, A. Ramazani, S.K. Ardestani, A. Shafiee, Coumarin-based bioactive compounds: facile synthesis and biological evaluation of coumarin-fused 1,4-thiazepines. Chem. Biol. Drug Des. 78, 580–586 (2011)
A. Souldozi, A. Ramazani, N. Bouslimani, R. Welter, The reaction of (N-isocyanimino)triphenylphosphorane with dialkyl acetylenedicarboxylates in the presence of 1,3-diphenyl1,3-propanedione: a novel three-component reaction for the stereoselective synthesis of dialkyl (Z)-2-(5,7-diphenyl1,3,4-oxadiazepin-2-yl)-2-butenedioates. Tetrahedron Lett. 48, 2617–2620 (2007)
Z. Hosseinzadeh, A. Ramazani, N. Razzaghi-Asl, Anti-cancer nitrogen-containing heterocyclic compounds. Curr. Org. Chem. 22, 2256–2279 (2018)
A. Ramazani, A.T. Mahyari, M. Rouhani, A. Rezaei, A novel three-component reaction of a secondary amine and a 2-hydroxybenzaldehyde derivative with an isocyanide in the presence of silica gel: an efficient one-pot synthesis of benzo[b]furan derivatives. Tetrahedron Lett. 50, 5625–5627 (2009)
A. Ramazani, A.R. Kazemizadeh, Preparation of stabilized phosphorus ylides via multicomponent reactions and their synthetic applications. Curr. Org. Chem. 15, 3986–4020 (2011)
M. Khoobi, S. Emami, G. Dehghan, A. Foroumadi, A. Ramazani, A. Shafiee, Synthesis and free radical scavenging activity of coumarin derivatives containing a 2-methylbenzothiazoline motif. Arch. Pharm. 344, 588–594 (2011)
A.P. Taylor, R.P. Robinson, Y.M. Fobian, D.C. Blakemore, L.H. Jones, O. Fadeyi, Modern advances in heterocyclic chemistry in drug discovery. Org. Biomolecular Chem. 14, 6611–6637 (2016)
R. Aggarwal, G. Sumran, An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 205, 112652 (2020)
L.D. Rodrigues, D. Sunil, D. Chaithra, P. Bhagavath, 1,2,3/1,2,4-Triazole containing liquid crystalline materials: An up-to-date review of their synthetic design and mesomorphic behavior. J. Mol. Liq. 297, 111909 (2020)
R. Liu, L. Wu, H. Feng, F. Tang, H. Si, X. Yao, W. He, The study on the interactions of two 1,2,3-triazoles with several biological macromolecules by multiple spectroscopic methodologies and molecular docking. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 243, 118795 (2020)
S. Kumar, B. Sharma, V. Mehra, V. Kumar, Recent accomplishments on the synthetic/biological facets of pharmacologically active 1H–1,2,3-triazoles. Eur. J. Med. Chem. 212, 113069 (2021)
Z. Xua, Z. Shi-Jia, Y. Liu, 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 183, 111700 (2019)
K. Bozorov, J. Zhao, H.A. Aisa, 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 27, 3511–3531 (2019)
E. Bonandi, M.S. Christodoulou, G. Fumagalli, D. Perdicchia, G. Rastelli, D. Passarella, The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discovery Today 22, 1572–1581 (2017)
C.K. Jaladanki, S. Khatun, H. Gohlke, P.V. Bharatam, Reactive metabolites from thiazole-containing drugs: Quantum chemical insights into biotransformation and toxicity. Chem. Res. Toxicol. 34, 1503–1517 (2021)
S. Maddila, S. Gorle, S.B. Jonnalagadda, Drug screening of rhodanine derivatives for antibacterial activity. Expert Opinion Drug Discovery. 15, 203–229 (2020)
L.J. Yin, A.K.D.B.A. Kamar, G.T. Fung, C.T. Liang, V.R. Avupati, Review of anticancer potentials and structure-activity relationships (SAR) of rhodanine derivatives. Biomed. Pharmacother. 145, 112406 (2022)
A.K.D.B.A. Kamar, L.J. Yin, C.T. Liang, G.T. Fung, V.R. Avupati, Rhodanine scaffold: A review of antidiabetic potential and structure–activity relationships (SAR). Med. Drug Discovery 15, 100131 (2022)
S.K. Manjal, R. Kaur, R. Bhatia, K. Kumar, V. Singh, R. Shankar, R. Kaur, R.K. Rawal, Synthetic and medicinal perspective of thiazolidinones: A review. Bioorg. Chem. 75, 406–423 (2017)
F.M. Aqlan, A.S. Al-Bogami, N.F. Alqahtani, M.Y. Wani, S.A. Khan, Thiazolidinone: A structural motif of great synthetic and biological importance. J. Mol. Struct. 1250, 131771 (2022)
L.M. Nainwal, S. Tasneem, W. Akhtar, G. Verma, M.F. Khan, S. Parvez, M. Shaquiquzzaman, M. Akhter, M.M. Alam, Green recipes to quinoline: A review. Eur. J. Med. Chem. 164, 121–170 (2019)
H. Mustroph, Correspondence on “cyanine dyes containing quinoline moieties: History, synthesis, optical properties and applications.” Chem. A Euro. J. 28, e2021037 (2022)
L. Xing-Hai, F. Yue-Ming, X. Feng, Z. Rui-Rui, S. Zhong-Hua, T. Chen-Xia, W. Jian-Quan, X. Tian-Ming, H. Hong-Ying, Synthesis and in vivo fungicidal activity of some new quinoline derivatives against rice blast. Pest Manag. Sci. 73, 1900–1907 (2017)
V. Sharma, R. Das, D.K. Mehta, S. Gupta, K.N. Venugopala, R. Mailavaram, A.B. Nair, A.K. Shakya, P.K. Deb, Recent insight into the biological activities and SAR of quinolone derivatives as multifunctional scaffold. Bioorg. Med. Chem. 59, 116674 (2022)
R. Kaura, K. Kumar, Synthetic and medicinal perspective of quinolines as antiviral agents. Eur. J. Med. Chem. 215, 113220 (2021)
B.S. Matada, R. Pattanashettar, N.G. Yernale, A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 32, 115973 (2021)
M. Dib, H. Ouchetto, K. Ouchetto, A. Hafid, M. Khouili, recent developments of quinoline derivatives and their potential biological activities. Curr. Org. Synth. 18, 248–269 (2021)
C. B. Pradeep Kumar, M. S. Raghu, B. S. Prathibha, M. K. Prashanth, G. Kanthimathi, K. Yogesh Kumar, L. Parashuram, F. A. Alharthi, Discovery of a novel series of substituted quinolines acting as anticancer agents and selective EGFR blocker: Molecular docking study. Bioorganic Med. Chem. Lett. 44 (2021) 128118.
P. Zajdel, K. Marciniec, A. Maslankiewicz, K. Grychowska, G. Sata1a, B. Duszynska, T. Lenda, A. Siwek, G. Nowak, A. Partyka, D. Wrobel, M. Jastrzebska-Wiesek, A. J. Bojarski, A. Weso1owska, M. Paw1owski, Antidepressant and antipsychotic activity of new quinoline- and isoquinoline sulfonamide analogs of aripiprazole targeting serotonin 5-HT1A/5-HT2A/5-HT7 and dopamine D2/D3 receptors. Euro. J. Med. Chem. 60 (2013) 42–50.
S. Maddila, N. Kerru, S.B. Jonnalagadda, Synthesis and antimicrobial evaluation of novel pyrano[2,3-d]-pyrimidine bearing 1,2,3-triazoles. Chem. Data Collect. 28, 100486 (2020)
N. Kerru, L. Gummidi, S. Maddila, K.K. Gangu, S.B. Jonnalagadda, A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 25, 1909 (2020)
S. Maddila, M. Momin, S. Gorle, L. Palakondu, S.B. Jonnalagadda, Synthesis and antioxidant evaluation of novel phenothiazine linked substitutedbenzylideneamino-1,2,4-triazole derivatives. J. Chil. Chem. Soc. 60(2), 2774–2778 (2015)
N. Kerru, V.H.S.S. Bhaskaruni, R. Kishore, S. Maddila, S.B. Jonnalagadda, Synthesis and antioxidant evaluation of a new class of thienopyrimidine-rhodanine hybrids. Lett. Drug Des. Discovery 15, 118–126 (2018)
S. Gorle, S. Maddila, S.N. Maddila, K. Naicker, M. Singh, P. Singh, S.B. Jonnalagadda, Synthesis, molecular docking study and in vitro anticancer activity of tetrazole linked benzochromene derivatives. Anticancer Agents Med. Chem. 17, 464–470 (2017)
S. Gorle, S. Maddila, S. Chokkakula, P. Lavanya, M. Singh, S.B. Jonnalagadda, Synthesis, biological activity of pyrimidine linked with morpholinophenyl derivatives. J. Heterocycl. Chem. 53, 1852–1858 (2016)
S. Maddila, S. Gorle, N. Seshadri, P. Lavanya, S.B. Jonnalagadda, Synthesis, antibacterial and antifungal activity of novel benzothiazole pyrimidine derivatives. Arab. J. Chem. 9, 681–687 (2016)
K. Suryanarayana, S. Maddila, N. Kerru, S.B. Jonnalagadda, Design, synthesis, docking study and biological evaluation of novel thieno[2,3- d]-pyrimidine tethered 1,2,3-triazole scaffolds. J. Mol. Struct. 1250, 131713 (2022)
K. Suryanarayana, A.R. Robert, N. Kerru, T. Pooventhiran, R. Thomas, S. Maddila, S.B. Jonnalagadda, Design, synthesis, anticancer activity and molecular docking analysis of novel dinitrophenylpyrazole bearing 1,2,3-triazoles. J. Mol. Struct. 1243, 130865 (2021)
K. Gopaul, N.A. Koorbanally, Synthesis and structure elucidation of a series of chloroquinoline-2-chalcones by the Doebner-Miller reaction. Magn. Reson. Chem. 54, 677–683 (2016)
N. Kerru, S.N. Maddila, S. Maddila, S. Sobhanapuram, S.B. Jonnalagadda, Synthesis and antimicrobial activity of novel thienopyrimidine linked rhodanine derivatives. Can. J. Chem. 97, 94–99 (2019)
J. M. Van, G. J. L. Kaspers, Cloos, Cell sensitivity assays: the MTT assay. J. Cancer Cell Culture: Meth. Mol. Biol. 731, 237–245 (2011).
P. W. Sylvester, Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Drug Des. Discov. Meth. Mol. Biol. 16, 157–168 (2011).
M.M. Ghor, F.A. Raja, S.I. Alqasoumi, A.M. Alafeefy, S.A. Aboulmagd, Synthesis of some new pyrazolo-[3,4-d]-pyrimidine derivatives of expected anticancer and radioprotective activity. Eur. J. Med. Chem. 45, 171–178 (2010)
F. Zafar, A. Gupta, T. Thangeval, K. Khatana, A.S. Alhaji, A. Ghosal, Physicochemical and pharmacokinetic analysis of anacardic acid derivatives. ACS Omega 5, 6021–6030 (2020)
Acknowledgements
The authors are very grateful to the University of KwaZulu Natal, Durban, South Africa, and GITAM Deemed to be University, Bengaluru and Visakhapatnam, India for financial and research support.
Funding
Open access funding provided by University of KwaZulu-Natal.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suryanarayana, K., Gangu, K.K., Kerru, N. et al. Synthesis of a novel chloroquinoline, rhodanine encompassed 1,2,3-triazole scaffolds and molecular docking evaluation of their cytotoxicity. J IRAN CHEM SOC 20, 2643–2655 (2023). https://doi.org/10.1007/s13738-023-02862-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02862-2