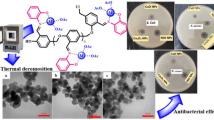
Abstract
4-{[(3-Hydroxynaphthalen-2-yl)imino]methyl}benzene-1,2,3-triol Schiff-base, abbreviated as ligand (L), was synthesized and additionally, its polymer was obtained through oxidative polycondensation and named as poly-4-{[(3-hydroxynaphthalen-2-yl)imino]methyl}benzene-1,2,3-triolpoly-ligand (PL). The ammonium persulfate (NH4)2S2O8 was used as an oxidant in this reaction. The metal complexes of PL were synthesized in the presence of various metal ions such as Cu2+, Hg2+, Ni2+, Pb2+,and Zn2+. The synthesized structures of PL and metal complexes were confirmed by FTIR, UV–vis, 1H and 13C NMR, and elemental analysis. Moreover, SEM and TGA analyses were performed for characterization. In this study, the anti-oxidant, anti-bacterial and catalytic properties of PL and its metal complexes were investigated. For anti-oxidant properties, 2,2-diphenyl-1-picryl-hydrazyl (DPPH) detection methods were studied. Also, for anti-bacterial activities they were tested against various bacteria using the minimum inhibitory concentration (MIC) method. It was observed that [Ni(PL)2] and [Pb(PL)2] metal complexes showed quite good anti-oxidant activities when they were compared with PL at its highest concentration of 100 ppm. The anti-bacterial activity results showed that [Hg(PL)2] had the highest MIC value of all the mentioned materials. The obtained catalytic activity results revealed that the synthesized poly-ligand metal [M(PL)2] complexes could be considered as an alternative catalyst to remove various organic pollutants from the aqueous environment. From this point of view, it is possible to say that [M(PL)2] complexes synthesized within the scope of the study could be used as polymeric agents to reduce environmental pollution.
Graphical abstract









Similar content being viewed by others
Data availability
The data that support the fndings of this study are available from the corresponding author, upon reasonable request.
References
Ajani OO, Obafemi CA, Nwinyi OC, Akinpelu DA (2010) Microwave assisted synthesis and antimicrobial activity of 2-quinoxalinone-3-hydrazone derivatives. Bioinorg Med Chem 18:214–221
Aslam MAS, Mahmood SU, Shahid M, Saeed A, Iqbal J (2011) Synthesis, biological assay in vitro and molecular docking studies of new Schiff base derivatives as potential urease inhibitors. Eur J Med Chem 46:5473–5479
Cui Z, Li Y, Ling Y, Huang J, Cui J, Wang R, Yang X (2010) New class of potent antitumor acylhydrazone derivatives containing furan. Eur J Med Chem 45:5576–5584
Asif M, Husain A (2013) Analgesic, anti-inflammatory, and antiplatelet profile of hydrazones containing synthetic molecules. J Appl Chem. https://doi.org/10.1155/2013/247203
Razaeivala M, Keypour H (2014) Schiff base and non-Schiff base macrocyclic ligands and complexes incorporating the pyridine moiety – The first 50 years. CoordChem Rev 280:203–253
Eren T, Kose M, Kurtoglu N, Ceyhan G, McKee V, Kurtoglu M (2015) An azo-azomethine ligand and its copper(II) complex: synthesis, X-ray crystal structure, spectral, thermal, electrochemical and photoluminescence properties. Inorg Chim Acta 430:268–279
Kocaeren AA, BahçeciŞenol D, Doğan F (2022) Optical sensor applications of carbazole derivative polymers containing different aliphatic groups against Fe3+ metal ion. Polym Sci Ser A 64:685–703
BahçeciŞenol D, Demir N, Kocaeren Aydın A (2022) Biological activity and optical sensor properties of green synthesis polymer. ChemSelect 7:e202202096
Kaya I, Daban S, Senol D (2021) Synthesis and characterization of Schiff base, Co (II) and Cu(II) metal complexes and poly(phenoxy-imine)s containing pyridine unit. Inorg Chim Acta 515:120040
Kaya I, Orta IE, Özdemir E, Senol D (2018) Synthesis, characterization, optimum reaction conditions, and some polymer–metal complexes of poly(phenoxy-imine)s containing furan ring. J Iran Chem Soc 15:35–46
Kaya İ, Yıldırım M, Aydın A, Şenol D (2010) Synthesis and characterization of fluorescent graft fluorene co polyphenol derivatives: the effect of substituent on solubility, thermal stability, conductivity, optical and electrochemical properties. React Funct Polym 70:815–826
Senol D, Kaya I (2017) Synthesis and characterizations of poly(phenoxy-imine)s via catalyzedoxidative polymerization by polymer–metal complex. Arab J Sci Eng 42:2381–2396
BahceciSenol D, Demir N (2022) Investigation of synthesis, characterization, and biological activities of water-soluble polymer with Horseradish peroxidase enzyme. Polym Sci Ser B 64:733–748
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Gwaram NS, Ali HM, Khaledi H, Abdulla MA, Hadi AHA, Lin TK, Ching CL, Ooi CL (2012) Antibacterial evaluation of some Schiff bases derived from 2-acetylpyridine and their metal complexes. Molecules 17:5952–5971
Manandhar S, Luitel S, Dahal RK (2019) In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J Tropical Med 5:1
Bedlovicova Z, Strapac I, Balaz M, Salayova A (2020) A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 25:3191
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302
Bast A, Haenen GR, Doelman CJ (1991) Oxidants and antioxidants: state of the art. Am J Med 91:2–13
Takata T, Shinohara K, Tanaka A, Hara M, Kondo JN, Domen K (1997) A highly active photocatalyst for overall water split-ting with a hydrated layered perovskite structure. J Photochem Photobiol A 106:45–49
Sarıoğlu Cebeci M, Selçuk F (2020) Photocatalytic dye removal and mineralization from wastewater. Academic Platform J Eng Sci 8:533–539
Kirchon A, Zhang P, Li J, Joseph EA, Chen W, Zhou H, Zhou C (2020) Effect of isomorphic metal substitution on the fenton and photo-fentondegradation of methylene blue using Fe-based metal−organic frameworks. ACS Appl Mater Interf 12:9292–9299
He Y, Ma Z, Junior LB (2020) Distinctive binary g-C3N4/MoS2heterojunctions with highly efficient ultrasonic catalytic degradation for levofloxacin and methylene blue. Ceram Int 46:12364–12372
Mahmoud AED, El-Maghrabi N, Mohamed Hosny M, Fawzy M (2022) Biogenic synthesis of reduced graphene oxide from Ziziphusspina-christi(Christ’s thorn jujube) extracts for catalytic, antimicrobial, and antioxidant potentialities. Environ Sci Pollu Res 29:89772–89787
Eltaweil AS, Abdelfatah AM, Hosny M, Fawzy M (2022) Biogenic synthesis of a Ag@Biocha rnanocomposite as an antimicrobial agent and photocatalyst for methylene blue degradation. ACS Omega 7:8046–8059
Blois MS (1985) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Clinical and Laboratory Standarts Institute (CLSI) (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard-Seventh Ed., M07-A7, Villanova PA, USA
Schiff H (1869) Ueber condensirte Harnstoffe. Justus Liebigs Annal Chem 150:193–200
Berber N, Arslan M (2020) Preparation and characterization of some Schiff base compounds. ADYU J Sci 10:179–188
Karatağ E (2023) Master Thesis Turkey Çanakkale Onsekiz Mart University. Çanakkale, Turkey
Bilici A, Dogan F, Yıldırım M, Kaya İ (2012) Tunable multicolor emission in oligo(4-hydroxyquinoline). J Phys Chem C 116:19934–19940
Mighani H (2020) Schiff Base polymers: synthesis and characterization. J Polym Res 27:168–186
Kocaeren Aydın A (2016) Synthesis and electrochromic performance of a novel polymer based on an oxidative polymer derived from carbazole and thiophene. J Polym Res 23:66–75
Saeed SES, Al-Harbi TM, Alhakimi AN, Abd El-Hady MM (2022) Synthesis and characterization of metal complexes based on aniline derivative Schiff base for antimicrobial applications and UV protection of a modified cotton fabric. Coatings 12:1181–1199
Fernandez ER, Manzano JL, Benito JJ, Hermosa R, Monte E, Criado JJ (2005) Thiourea, triazole and thiadiazine compounds and their metal complexes as antifungal agents. J Inorg Biochem 99:1558–1572
Polat G (2002) Synthesis and investigation of new thiourea derivatives and chelate compounds of Ni(II), Co(II) and Cu(II) ions. Master Thesis, Mersin University Institute of Science, Department of Chemistry
Emen FM (2002) Synthesis and characterization of new thiourea derivatives of 3d-transition metals and chelate compounds. Master Thesis, Mersin University Institute of Science, Department of Chemistry
Braun U, Richter R, Sieler J (1985) Kristall und molekül von tris(1.1-diethyl-3-benzoylthioharnstoff) Silber (I)-hydrogen sülfid. Z Anorg AllgChem 529:201–208
Arslan H, Külcü N, Flörke U (2003) Synthesis and characterization of copper(II), nickel(II) and cobalt(II) complexes with novel thiourea derivatives. Trans Metal Chem 28:816–819
Emen MF, Arslan H, Külcü N, Flörke U, Duran N (2005) Synthesis, characterization and antimicrobial activities of some metal complexes with N-(2-chloro-benzoyl)thiourea ligands: the crystal structure of fac-[CoL3] and cis-[PdL2]. Polish J Chem 79:1615–1626
Uğur D (2004) Synthesis and investigation of terephthalic acid-thiourea derivative ligands and some metal complexes of these ligands. Master Thesis, Mersin University, Graduate School of Natural and Applied Sciences, Department of Chemistry
Arslan H, Flörke U, Külcü N, Emen FM (2006) Crystal structure and thermal behaviour of copper(II) and zinc(II) complexes with N-pyrrolidine-N′-(2-chlorobenzoyl)thiourea. J Coord Chem 59:223–228
Kayhan E (2006) Synthesis, characterization, crystal structure and thermal behaviour of N’-(4-chlorobenzoyl)-N.N-di-n-butylthiourea and its nickel complex. Turk J Chem 30:429–440
Bal M (2010) Synthesis of new azo Schiff base type ligand and metal complexes, investigation of their spectroscopic and genotoxic properties. Master Thesis, KahramanmarasSutcu Imam University, Graduate School of Natural and Applied Sciences
Erdik E (2008) Spectroscopic methods in organic chemistry. Ankara University. Faculty of Science, Gazi Bookstore, Ankara
Smith PAS (1966) The chemistry of open chain organic nitrogen compounds, vol III. Benjamin, New York, pp 29–68
Wu C, Mosher BP, Zeng TJ (2006) One-step green route to narrowly dispersed copper nanocrystals. J Nanopart Res 8:965–969
Pourbasheer E, Morsali S, Banaei A, Aghabalazadeh S, Ganjali MR, ParvizNorouzi P (2015) Design of a novel optical sensor for determination of trace amounts of copper by UV/vis spectrophotometry in the real samples. J Ind Eng Chem 26:370–374
ChamjangaliM A, Soltanpanah S, Goudarzi N (2009) Development and characterization of a copper optical sensor based on immobilization of synthesized 1-phenyl-1,2-propanedione-2-oxime thiosemicarbazone on a triacetylcellulose membrane. Sens Actuat B 138:251–256
Valarmathy G, Subbalakshmi R, Sumathi R, Renganathan R (2020) Synthesis of Schiff base ligand from N-substituted benzenesulfonamide and its complexes: Spectral, thermal, electrochemical behavior, fluorescence quenching, in vitro-biological and in-vitro cytotoxic studies. J Mol Struct 1199:127029
Daravath S, Vamsikrishna N, Ganji N, Venkateswarlu K (2018) Synthesis, characterization, DNA binding ability, nuclease efficacy and biological evaluation studies of Co (II), Ni (II) and Cu (II) complexes with benzothiazole Schiff base. Chem Data Collect 17:159–168
YıldırımUçan S (2019) Synthesıs of Schiff base complexes and investigation of structures. Omer Halisdemir Univ J Eng Sci 8:621–629
Yıldız A, Genç Ö, Bekta GS (1997) Instrumental analysis methods. Hacettepe University Publications A-64, Ankara, p 506
Nishat N, Khan SA, Rasool R, Parveen S (2011) Synthesis spectral characterization and biocidal activity of thermally stable polymeric Schiff base and its polymer metal complexes. J Inorg Organomet Polym 21:673–681
Uçan YS, Uçan M, Mercimek B (2005) Synthesis and characterization of some tetradentate Schiff bases and their cobalt(II), nickel(II), copper(II), zinc(II), cadmium(II) and mercury(II) complexes. Synth React Inorg Met Org Nano-Metal Chem 35:417–421
Mokhles M (2001) Spectroscopic characterization of some tetradentate Schiff bases and their complexes with Ni(II), Cu(II) and Zn(II). J Chin Chem Soc 48:153–158
Radha VP, JoneKirubavathy S, Chitra S (2018) Synthesis, characterization and biological investigations of novel Schiff base ligands containing imidazoline moiety and their Co(II) and Cu(II) complexes. J Mol Struct 1165:246–258
Bilici A, Cogal Y, Gecibesler IH, Kaya I (2021) Enzyme catalyzed synthesis of water soluble mesalazine oligomers and evaluation of their efficiency in polypropylene stabilization. Polym Sci Ser B 63:710–721
Bilici A, Geçibesler IH, Coga Y, Kaya I (2017) Biocatalytic synthesis of a novel polyaniline derivative and its usage for polypropylene stabilization. Ind Eng Chem Res 56:9266–9274
Yüksel E, Bilici A, Geçibesler IH, Kaya I (2020) Synthesis and antioxidant activities of phenolic Schiff base monomers and polymers. Canadian J Chem 98:151–157
Baran NY, Saçak M (2018) Molecular weight monitoring of poly(2-[[4-(dimethylamino)benzylidene]amino]phenol) depending on polymerization conditions: investigation of thermal stability, antimicrobial properties, and conductivity enhancement. Adv Polym Technol 37:3007–3016
Kaya I, Emdi D, Saçak M (2009) Synthesis, characterization and antimicrobial properties of oligomer and monomer/oligomer-metal complexes of 2-[(pyridine-3-yl-methylene)amino]phenol. J Inorg Organomet Polym Mater 19:286–297
Kaya I, ÇulhaoğluS SenolD (2007) Synthesis, characterizatıon and antimicrobial properties of 4-[(4-hydroxybenzylidene) amino] phenol and its polymer. Chin J Polym Sci 25:461–472
Kaya I, Bilici A, Saçak M (2006) Synthesis, characterization, and antimicrobial properties of oligo-4-[(pyridine-3-yl-methylene) amino] phenol. J Appl Polym Sci 102:3327–3333
Kaya I, Bilici A (2007) Syntheses, structures, electric conduction, electrochemical properties and antimicrobial activity of azomethine monomer and oligomer based on 4-hydroxybenzaldehyde and 2-aminopyridine. Polimery 52:827–835
Baran NY, Saçak M (2017) Synthesis, characterization and molecular weight monitoring of a novel Schiff base polymer containing phenol group: Thermal stability, conductivity and antimicrobial properties. J Mol Struct 1146:104–112
Tweedy BG (1964) Plant extracts with metal ions as potential antimicrobial agents. Phytopatology 55:910–918
Nassar MY, Aly HM, Abdelrahman EA, MoustafaME, (2017) Synthesis, characterization, and biological activity of some novel Schiff bases and their Co (II) and Ni (II) complexes: a new route for Co3O4 and NiO nanoparticles for photocatalytic degradation of methylene blue dye. J Mol Struct 1143:462–471
Bartyzel A (2018) Synthesis, thermal behaviour and some properties of CuII complexes with N, O-donor Schiff bases. J Therm Anal Calorim 131:1221–1236
Gupta KC, Sutar AK (2007) Polymer anchored Schiff base complexes of transition metal ions and their catalytic activities in oxidation of phenol. J MolCatal A 272:64–74
Lin C, Fu J, Liu S (2019) Facile preparation of Au nanoparticle-embedded polydopamine hollow microcapsule and its catalytic activity for the reduction of methylene blue. J MacromolSci Part A: Pure Appl Chem 56:1104–1113
Xiong L, Huang R, Chai H, Yu L, Li CM (2020) Facile synthesis of Fe3O4@Tannic Acid@Aunanocomposites as a catalyst for 4-nitrophenol and methylene blue removal. ACS Omega 5:20903–20911
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Şenol Bahçeci, D., Aydın Kocaeren, A., Demir, N. et al. Anti-bacterial, anti-oxidant activities of polymer-based metal complexes and their catalyst effects in presence of H2O2. Iran Polym J 33, 141–156 (2024). https://doi.org/10.1007/s13726-023-01249-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-023-01249-7