Abstract
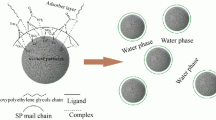
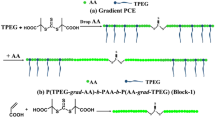
Acrylic acid–isobutylene polyethylene glycol (AA-TPEG) copolymers are typical of polycarboxylate superplasticizers (PCEs). AA-TPEG copolymers are prepared via free-radical polymerization with potassium persulfate as the initiator. The obtained copolymers were characterized by gel permeation chromatography (GPC) and infrared spectra (FTIR). The GPC method can break through the former limitations of the instruments and receive instantaneous unreacted and instantaneous monomer concentrations and not the initial monomer feeds. Since TPEG monomer is highly bulky, the common calculation methods for determining monomer reactivity ratios in copolymerization based on terminal copolymerization equation are not suitable. However, this study created non-linear least squares curve fitting of terminal copolymerization equation (NLLSQ-T) and penultimate copolymerization equation (NLLSQ-P) methods, which used Python’s NumPy, SciPy, and SymPy libraries to generate code and did numerical computations, bringing greater accuracy of monomer reactivity ratios. The monomer reactivity ratios were calculated with Fineman–Ross, Kelen–Tüdös, YBR, NLLSQ-T, and NLLSQ-P methods and found to be r AA = 10.888, r′ AA = 1.131, r TPEG = 0.012, and r′ TPEG = 0.042 for AA-TPEG copolymers. Moreover, this study also explored specific copolymerization behavior of similar structure of copolymers with steric hindrance under penultimate copolymerization equation, such as dependence of the mole fractions in the copolymer on the mole fractions of unreacted monomers in solution, variation of copolymer compositions with conversion and sequence length distribution. The fluidity and flow loss of pastes containing PCEs were investigated, and the appropriate PCEs dosages resulted in a better workability of cement pastes.










Similar content being viewed by others
References
Hirata T (1981) Cement dispersant. JP Patent 84(2022):S59–018338
Li C-Z, Feng N-Q, Chen R-J (2005) Effects of polyethylene oxide chains on the performance of polycarboxylate-type water-reducers. Cement ConcRes 35:867–873
Uchikawa H, Hanehara S, Sawaki D (1997) The role of steric repulsive force in the dispersion of cement particles in fresh paste prepared with organic admixture. Cement Concr Res 27:37–50
Liu X, Wang Z, Zhu J, Zheng Y, Cui S, Lan M, Li H (2014) Synthesis, characterization and performance of a polycarboxylate superplasticizer with amide structure. Colloid Surf A Physicochem Eng Asp 448:119–129
Alonso M, Palacios M, Puertas F (2013) Compatibility between polycarboxylate-based admixtures and blended-cement pastes. Cement Concr Compos 35:151–162
Werfel TA, Swain C, Nelson CE, Kilchrist KV, Evans BC, Miteva M, Duvall CL (2016) Hydrolytic charge-reversal of PEGylated polyplexes enhances intracellular un-packaging and activity of siRNA. J Biomed Mater Res A 104:917–927
Liu B, Liu D-T, Li S-H, Sun G-P, Cui D-M (2016) High trans-1,4 (co)polymerization of β-myrcene and isoprene with an iminophosphonamide lanthanum catalyst. Chin J Polym Sci 34:104–110
Ziaee F, Nekoomanesh M (1998) Monomer reactivity ratios of styrene-butyl acrylate copolymers at low and high conversions. Polymer 39:203–207
Moskowitz JD, Wiggins JS (2016) Semibatch RAFT copolymerization of acrylonitrile and N-isopropylacrylamide: effect of comonomer distribution on cyclization and thermal stability. Polymer 84:311–318
Dréan M, Guégan P, Jérôme C, Rieger J, Debuigne A (2016) Far beyond primary poly(vinylamine)s through free radical copolymerization and amide hydrolysis. Polym Chem 7:69–78
Kelen T, Tüdős F (1974) A new improved linear graphical method for determing copolymerization reactivity ratios. React Kinet Catal Lett 1:487–492
Fineman M, Ross SD (1950) Linear method for determining monomer reactivity ratios in copolymerization. J Polym Sci 5:259–262
Consolante V, Maric M, Penlidis A (2012) Routes to carboxylic acid functional acrylonitrile copolymers via N-tert-butyl-N-(1-diethylphosphono-2,2-dimethylpropyl) free nitroxide based nitroxide-mediated polymerization. J Appl Polym Sci 125:3963–3976
Mayo FR, Lewis FM (1944) Copolymerization. I. A basis for comparing the behavior of monomers in copolymerization; the copolymerization of styrene and methyl methacrylate. J Am Chem Soc 66:1594–1601
Yezrielev A, Brokhina E, Roskin YS (1969) An analytical method for calculating reactivity ratios. Polym Sci USSR 11:1894–1907
Joshi R, Joshi S (1971) A new analytical solution of the binary copolymer composition equation and suggested procedure for deriving the monomer reactivity ratios. J Macromol Sci Chem 5:1329–1338
Tüdos F, Kelen T, Földes-Berezsnich T, Turcsanyi B (1976) Analysis of linear methods for determining copolymerization reactivity ratios. III. Linear graphic method for evaluating data obtained at high conversion levels. J Macromol Sci Chem 10:1513–1540
Tidwell PW, Mortimer GA (1965) An improved method of calculating copolymerization reactivity ratios. J Polym Sci Part A General Papers 3:369–387
Lateulade A, Grassl B, Dagron-Lartigau C, François J (2006) Radical copolymerization of N-vinylcarbazole and p-bromostyrene: determination of monomer reactivity ratios by SEC-multidetection. Polymer 47:2280–2288
Pal S, Banoth B, Rahithya G, Dhawan A, De P (2012) Copolyperoxides of 2-(acetoacetoxy) ethyl methacrylate with methyl methacrylate and styrene; synthesis, characterization, thermal analysis, and reactivity ratios. Polymer 53:2583–2590
Singh A, Kamal M, Singh D (2011) Diphenylselenonium 2,3,4,5-tetraphenylcyclopentadienylide-initiated polymerization of styrene and acrylonitrile: synthesis, characterization, reactivity ratios, and thermal properties. J Appl Polym Sci 121:1052–1058
Suga T, Aoki K, Yashiro T, Nishide H (2016) “Click” incorporation of radical/ionic sites into a reactive block copolymer: afacile and on-demand domain functionalization approach toward organic resistive memory. Macromol Rapid Commun 37:53–59
Xu A, Yuan WZ, Zhao J, Li H, Zhang H, Zhang Y (2012) Copolymerizations of tetrafluoroethylene and perfluoropropylvinyl ether in supercritical carbon dioxide: polymer synthesis, characterization, and thermal properties. J Appl Polym Sci 124:1785–1795
Çanak TÇ, Hamuryudan E, Serhatlı İE (2013) Synthesis and characterization of perfluorinated acrylate–methyl methacrylate copolymers. J Appl Polym Sci 128:1450–1461
Yan Y, Lu N, Cui J, Zhang J (2012) Synthesis of fluorescent poly(1-vinylimidazole-co-(1-pyrene) methyl 2-methyl-2-propenote) and determination of monomer reactivity ratios. J Appl Polym Sci 125:2867–2873
Chen W, Zheng H, Guan Q, Teng H, Zhao C, Zhao C (2016) Fabricating a flocculant with controllable cationic microblock structure: characterization and sludge conditioning behavior evaluation. Indust Eng Chem Res 55:2892–2902
Shekh MI, Patel DM, Patel KP, Patel RM (2016) Electrospun nanofibers of poly(NPEMA-co-CMPMA): used as heavy metal ion remover and water sanitizer. Fibers Polym 17:358–370
Biswal T, Samal R, Sahoo PK (2012) Microwave-assisted preparation of poly(2-EHA-co-ST) copolymer and poly(2-EHA-co-ST)/MMT nanocomposite. J Appl Polym Sci 125:1467–1475
Lartey M, Gillissen M, Adzima BJ, Takizawa K, Luebke DR, Nulwala HB (2013) Synthesis and reactivity ratios of regioisomeric vinyl-1,2,3-triazoles with styrene. J Polym Sci Part A Polym Chem 51:3359–3364
Deb PC (2005) Non-uniqueness of penultimate model reactivity ratios and treatment of kinetic data. Polymer 46:6235–6242
Lewis FM, Mayo FR (1945) Precise method for isolation of high polymers. Indust Eng Chem Anal Ed 17:134–136
Skeist I (1946) Copolymerization: the composition distribution curve. J Am Chem Soc 68:1781–1784
Ham GE (1954) Reactivity of polar monomers in copolymerization. J Polym Sci 14:87–93
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Wang, Z., Ren, J. et al. Polycarboxylate superplasticizers of acrylic acid–isobutylene polyethylene glycol copolymers: monomer reactivity ratios, copolymerization behavior and performance. Iran Polym J 25, 549–557 (2016). https://doi.org/10.1007/s13726-016-0446-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-016-0446-4