Abstract
The present work reports the effect of niobium addition on a Fe–7wt.%Al–0.35wt.%C-based low-density steel. A hot rolling temperature of 1100 °C was selected to carry out rolling in the intercritical (ferrite + austenite) region. The phases formed on adding Nb were predicted by thermodynamic calculations using ThermoCalc. All the alloys exhibited a significant (20% or more) tensile elongation. Niobium addition also resulted in a significant increase in strength and hardness of the hot-rolled steel. About 80% increase in the yield strength is observed as the Nb content increases from 0.2 to 1.0 wt.%. Nb carbides present at rolling temperature resist grain growth and lead to improved mechanical properties. This study demonstrates the advantages of adding Nb to Fe–Al–C-based low-density steels. Earlier attempts to add Nb to low-density steels resulted in very low ductility. This may be related to their susceptibility to hydrogen embrittlement. Melting of high aluminum low-density steels in a controlled atmosphere may lead to a considerable improvement in mechanical properties. Further, it is also important to maintain a high C/Nb ratio to avoid the formation of Laves phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The density of steel can be reduced by the addition of aluminum [1,2,3,4]. Each wt.% addition of Al gives about 40 MPa increase in strength by solid solution strengthening [1,2,3,4,5] and a density reduction of about 1.5% [3,4,5,6,7]. Aluminum addition also leads to an improved corrosion resistance [1, 8]. Because of their low-density, high specific strength, and good corrosion resistance, these steels are considered potential structural materials in thermal power plants and petrochemical industry [1,2,3,4,5,6,7,8,9,10,11,12,13]. Reducing the weight of an automobile by 10% can hike the fuel economy by 6–8%, and therefore, these materials have applications in the automotive industry [1,2,3,4, 10, 11]. Further, they may also have potential defense applications [12]. We have earlier reported properties of Fe–7wt.%Al alloys with carbon addition [5, 7]. Here, we report the effect of Nb addition on microstructure and mechanical properties of a Fe–7wt.%Al–0.35wt.%C alloy (all compositions are in wt.%). In order to predict the formation of phases on Nb addition, thermodynamic calculations were performed using ThermoCalc software and compared with the experimental results. Earlier attempts to add Nb to low-density steels resulted in very low ductility [13,14,15,16]. This may be related to their susceptibility to hydrogen embrittlement. All the alloys studied in the present work exhibited significant (20% or more) tensile elongation. It is suggested that the melting of high aluminum low-density steels in a controlled atmosphere may be necessary to achieve improved mechanical properties.
Experimental Procedure
High-purity raw materials such as iron, aluminum, graphite, and niobium were used for preparing steel pancakes of desired compositions. Non-consumable DC arc melting process with a thoriated tungsten electrode was used for melting the raw materials. The melting chamber was evacuated to 1 × 10−3 mbar and refilled with argon. The process of evacuation and refilling was performed twice. During melting, the argon gas pressure was maintained at 600 mbar. The arc was initiated without direct contact between the tungsten electrode and the charge material. A current of 850–900 amperes and voltage of 18–20 V were used during melting. The alloy was cast as a pancake of 100 mm diameter and 10 mm thickness in a water-cooled copper crucible. For each composition, the pancake was melted three times with periodic reversal of the pancake top. Pancakes were checked for defects by Gamma-ray radiography using a 5-Curie Co60 radioactive source. The amount of Al and Nb present in the pancakes was determined by wet chemical analysis. A Leco CS-444 was used to measure the carbon content. The pancake samples were soaked in a furnace for 1 h at a temperature of 1100 °C. A Demag rolling mill was used to hot-roll the samples to a sheet of 2 mm thickness. A reduction of 1 mm in thickness was given to the samples during each pass. After every three passes, the samples were reheated to 1100 °C.
As-cast as well as hot-rolled samples were polished to 0.5-µm finish for microstructural studies. The polished samples were etched with a solution of 33% HNO3 + 1% HF + 33% CH3COOH + 33% H2O by volume for microstructural observation. Both as-polished and etched samples were studied in a Leo 440I scanning electron microscope (SEM). The grain size of hot-rolled alloys was determined from optical micrographs using the linear intercept method. The phases present in polished low-density steel samples were identified by Philips x-ray diffractometer using a monochromatic CuKα (λ = 15.40562 nm) radiation at a scan rate of 1° min−1. The electron probe microanalysis (EPMA) studies were carried out on polished samples to determine the matrix and precipitate compositions and the distribution of the elements present in the steel. The volume fraction of precipitates present in steels was measured using the Biovis Materials Plus Software on SEM images. The average volume fraction of the precipitates on ten SEM images at different locations of each alloy is reported. Thermodynamic calculations were performed to identify phases present at various temperatures and to study phase transformations in the alloys using TCFE6 database of ThermoCalc software. The density of the hot-rolled alloy sheets was measured based on Archimedes principle using a density measuring kit of Sartorius make. The average of five independent measurements was recorded.
The hardness of metallographic samples was measured in a Leco LV700 Vickers hardness testing machine using a 30-kg load and a dwell time of 0.166 min. Microhardness of individual phases was also measured using an Economet VH 1D microhardness machine with 100 g load. Tensile tests were carried out at an initial strain rate of 1.6 × 10−5 min−1 using a universal testing machine (Instron 5500R) on samples of hot-rolled sheet conforming to ASTM E-8M. The tensile-tested alloy sample’s fracture surfaces were observed in the SEM.
Results
Chemical compositions of the alloys melted are given in Table 1. Radiography studies confirmed that the pancakes are free from internal cracks and porosity. The density of hot-rolled alloys varies in the range of 7159.68 to 7181.92 kg/m3. X-ray diffraction revealed the presence of Fe3AlC0.5 (κ-carbide) (Fig. 1) and ferrite (α) in all the samples. The alloys exhibited a dendritic microstructure with a significant amount of precipitates (Fig. 2). Two types of precipitates (gray and bright) are observed (Fig. 3). As the niobium content increases, the volume fraction of the bright precipitates increases. The bright phase is rich in Nb and C. The gray phase is identified as Fe3AlC0.5. In alloys containing up to 0.7 wt.% Nb, the backscattered electron (BSE) SEM micrographs reveal globular bright precipitates. In the 1 wt.% Nb alloy, thin needle-shaped bright precipitates are observed.
Figure 4 shows an equilibrium phase diagram of Fe–7Al–0.35C varying Nb content predicted using ThermoCalc software. The various phases and their amounts present in these steels containing 0 to 1 wt.% niobium at different temperature as calculated by using ThermoCalc are shown in Fig. 5a–e. The solidification of the alloy starts at 1484 °C. The NbC precipitation may start from the liquid, while the κ-carbide (Fe3AlC0.5) forms at 812 °C. Based on the Nb content, the NbC formation temperature changes and for the content 0.2 to 1 wt.% of Nb, the NbC formation temperature is in the range of 1430 to 1462 °C. The austenite phase is observed between 1410 and 810 °C. At room temperature ferrite (α), κ-carbide and NbC precipitates are the stable phases. Since the solubility of NbC is high in austenite [17, 18], further NbC precipitation occurs during the transformation from austenite to ferrite. From Fig. 4, we can see that the eutectic occurs at 0.083 wt.% of Nb at 1405 °C for Fe–7Al–0.35C steel. For these steel compositions with 0.083wt.%Nb and above NbC, precipitate formation starts from liquid (Fig. 5). At lower levels of Nb (< 0.083 wt.%), the NbC precipitate formation starts from austenite. At 1 wt.% Nb, the niobium carbide formed is very coarse and is in the order of 5–6 μm (Figs. 2d, 3d). Similar observations have been reported for steels containing high carbon and high niobium [18,19,20].
The pancakes were successfully hot-rolled from 10 to 2 mm thickness. Figure 6 shows the optical microstructures of the hot-rolled steel samples with different Nb contents. All samples exhibit partially recrystallized grains. As the Nb content increases, the average grain size decreases from about 320 to about 80 µm (Table 2). The partially recrystallized grains could also be observed in the backscattered electron (BSE) SEM micrographs of the hot-rolled steels (Fig. 7). In the alloy with 1%Nb, the morphology of Nb carbides changes from needle to globular after hot rolling. The EPMA analysis of the matrix and precipitates is summarized in Table 2. No Nb could be detected in the matrix and gray precipitates. The bright precipitates are found to be enriched in Nb and C (Fig. 8). Based on ThermoCalc results, these may be NbC precipitates. The volume fraction of both these precipitates is given in Table 3. The results (Table 3) show that the volume fraction of NbC is very small (< 5%) in all the alloys. Thus, its peaks could not be detected by XRD.
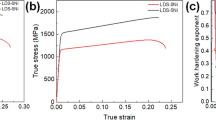
Figure 9 shows engineering stress–strain curves of all the alloys in the hot-rolled condition. The plot of logarithmic stress versus logarithmic plastic strain in the uniform deformation region is shown in Fig. 10. The values of strain hardening exponent ‘n’ and strength coefficient ‘k’ were determined assuming a power law relationship (σ = kεn) between logarithmic stress and logarithmic strain. The results are presented in Table 4. The strain hardening exponent remains largely unaffected by Nb addition; however, the strength coefficient increases from 798 to 1344 MPa (Fig. 10; Table 4). The hardness increases with increase in Nb content (Table 4). Further, yield strength and tensile strength also increase with the increase in Nb content (Fig. 9; Table 5). A significant increase (about 80%) in the yield strength is evident on increasing Nb content from 408 MPa at 0.2 wt.% Nb to 735 MPa at 1.0 wt.% Nb. This is accompanied by a decrease in tensile elongation from 30 to 20%. The 0.2 wt.% Nb alloy exhibited cleavage failure, while the alloys with higher Nb content exhibited mixed mode features comprising transgranular cleavage and ductile dimples (Fig. 11).
Discussion
A microstructure consisting of niobium carbide and Laves phases in the ferrite matrix has been reported for Fe–(7.8 to 8.5 Al) alloy containing different carbon and niobium [13, 14]. The presence of Laves phases is highly detrimental as it can lead to a loss in ductility. In the present study, no Laves phases are observed. This may be ascribed to the much higher C/Nb ratio maintained in the present study (1.75 to 0.35) as compared to value (0.06) reported in the literature [13, 14]. At room temperature, the type of the phases (ferrite (α), κ-carbide and NbC precipitates) predicted by the ThermoCalc (Fig. 5) agrees well with the type of phases from the experimental results (Fig. 8; Tables 2, 3). The volume fraction of the precipitates formation predicted by phase diagram is low compared to the volume fraction measured from micrographs (Table 3). The reason for the difference may be that the ThermoCalc database is not updated completely. Nevertheless, it is following a similar trend. Phases formed on Ti additions to Fe–7Al–0.35C alloy have also been successfully predicted [21] using ThermoCalc.
The alloys in the present work could easily be hot-rolled without any cracking. In contrast, Fe–Mn–Al–C alloys with similar Al and C contents have been reported to crack on hot rolling [22,23,24]. This may be due to intermediate heating of samples after every three passes during rolling practiced in the present work. This practice also ensures a more homogeneous microstructure and avoids the significant banding reported in earlier works [22,23,24].
The microstructure is significantly refined on hot working (Figs. 6, 7). As the niobium content increases, the ferrite grain size in the hot-rolled steels decreases significantly. This is because of the formation of fine niobium carbides at the ferrite boundaries. The rolling temperature of 1100 °C was in the intercritical (ferrite + austenite) region [24,25,26] where the dissolution of κ-carbides as well as part of NbC takes place in the austenite. This agrees well with the prediction of phases from the equilibrium diagram calculated by ThermoCalc (Figs. 4, 5). This is also supported by the reported stability of niobium carbides above 1200 °C [27,27,, 28]. In conventional steels, dynamic recrystallization of austenite is delayed by the addition of strong carbide formers such as Nb [29, 30]. On similar lines, the addition of niobium to Fe–7Al–0.35C may be expected to retard dynamic recrystallization in the present low-density steels during hot rolling. As discussed above, the precipitation of NbC occurs well above the hot rolling temperature. In the case of alloy with 0.2 wt.% Nb, the low volume fraction (0.86%) of NbC particles has resulted in coarse grain size (320 µm). This is attributed to the insufficient number of NbC particles present. As the Nb content is increased to 1 wt.%, there is a fourfold increase in the volume fraction (to 3.42%) of NbC precipitates. This restricts grain growth (Fig. 6) leading to fine grain size (80 µm). The size of NbC precipitates is in the range of 0.3 to 6 μm which may retard the grain growth. The EPMA line scan did not detect niobium in the matrix or in the κ-carbide precipitates. As the Nb content increases, more carbon is consumed in the formation of niobium carbide and subsequently volume fraction of the perovskite carbide decreases (Table 3).
The density of the alloys varies in the range of 7159.68 to 7181.92 kg/m3. These low-density steels show a reduction in density of ~ 10% compared to the conventional steel (7874.0 kg/m3). Both strength and hardness increase with an increase in niobium content. This may be attributed to the increase in the volume fraction of fine and hard niobium carbide precipitates and the resulting grain refinement. The increase in the yield strength is almost 80% as we increase the Nb content from 0.2 to 1 wt.%. At the same time, there is a decrease in ductility from 30 to 20%. However, all the alloys exhibited better ductility than 13.5% tensile elongation reported [5] for hot-rolled Nb free steel with similar Al and C content. The strain hardening exponent observed in the present work is ~ 0.15 (Table 4). The drop in n value with an increase in Nb content is very marginal despite the increase in strength. This may allow good formability of the steel for various operations like deep drawing, bending, etc.
The tensile test results of the present work are compared with those reported for the Fe–Al-based alloys containing different carbon and niobium levels (Table 5). It can be observed that steels containing higher aluminum (8.5 and 10.5 wt.%) with carbon and different niobium contents have shown high strength but poor ductility [14, 15]. This may be because these steels are prone to environmental embrittlement due to the presence of Al [5, 9, 31]. The reduced ductility may also be attributed to the ordering effect [3, 5, 9, 10]. The Fe–7%Al–0.35%C alloy processed by air induction melting under flux cover has been reported to have exhibited only 13.5% elongation [5]. The improved mechanical properties obtained in the present work are also attributed to the use of a controlled atmosphere during melting. It has also been demonstrated that a range of tensile properties is attainable by varying niobium content to Fe–7Al–0.35C steel. Use of controlled atmosphere appears critical from the present results. Melting process such as vacuum induction melting and vacuum arc melting are well established industrially and hence can be practiced for producing low-density steels containing carbon and niobium on a tonnage scale.
It is well known that coarse-grained ferritic steels mainly fracture by cleavage [32]. The resistance to cleavage is mainly provided by grain boundaries [32, 33]. Stress at the crack tip increases with the reciprocal root of the grain diameter. The plastic strain needed for fracture of a grain increases with decreasing grain size. This may be explained by a large number of dislocation pileup at a grain boundary which leads to coarse cleavage fracture [32]. This is observed in the steel with 0.2 wt.% Nb (Fig. 11a). A mixed mode fracture surface is exhibited by the alloys with 0.4 to 1 wt.% Nb which consists of finer dimples and shorter cleavage steps (Fig. 11b–d). This is attributed to the finer grain size displayed by the alloy with higher Nb content (Fig. 6; Table 2). Further, a large number of fine precipitates (NbC and κ-carbides) effectively reduce the dislocation pileup length, thereby reducing the size of the cleavage steps. Though the 0.2 wt.% Nb steel exhibited cleavage failure (Fig. 11a), it exhibits adequate ductility (30%). The volume fraction of hard niobium carbide increases with Nb content (Table 3), which leads to the reduction in ductility from 30 to 20%.
Conclusions
-
1.
The Fe–7Al–0.35C-based low-density steels containing 0.2% to 1% niobium could be successfully hot-rolled without showing any evidence of cracking or band formation. After 80% reduction, all the samples exhibited partially recrystallized grains.
-
2.
The alloys exhibited a ferritic matrix (α) containing Fe3AlC0.5 (κ-carbide) and NbC. The volume fraction of NbC precipitates increases with an increase in niobium content. The phases predicted using ThermoCalc agree well with the experimental results.
-
3.
A significant increase in strength and hardness is observed on increasing Nb content. This is attributed to the cumulative contribution from the increase in the volume fraction of the fine NbC carbides and the resulting grain refinement. All the compositions exhibit significant (> 20%) tensile elongation.
-
4.
It is also demonstrated that the use of a controlled atmosphere during melting may be necessary due to the presence of Al in these low-density steels. This may explain the low ductility reported on Nb addition for these steels. In addition, it is also important to maintain a high C/Nb ratio to avoid the formation of Laves phases.
References
S. Chen, R. Rana, A. Haldar, R.K. Ray, Current state of Fe–Mn–Al–C low density steels. Prog. Mater. Sci. 89, 345–391 (2017)
R. Rana, C. Lahaye, R.K. Ray, Overview of lightweight ferrous materials: strategies and promises. JOM 66(9), 1734–1746 (2014)
G. Frommeyer, E.J. Drewes, B. Engl, Physical and mechanical properties of iron–aluminium–(Mn, Si) lightweight steels. La Rev De Métallurgie-CIT 10, 1245–1253 (2000)
D.-W. Suh, N.J. Kim, Low-density steels. Scr. Mater. 68(6), 337–338 (2013)
V.V. Satya Prasad, S. Khaple, R.G. Baligidad, Melting, processing, and properties of disordered Fe–Al and Fe–Al–C based alloys. JOM 66(9), 1785–1793 (2014)
R. Rana, C. Liu, R.K. Ray, Low density low carbon Fe–Al ferritic steels. Scr. Mater. 68, 354–359 (2013)
S. Khaple, R.G. Baligidad, V.V. Satya Prasad, D.V.V. Satyanarayana, Microstructure and mechanical properties of Fe–7Al based lightweight steel containing carbon. Mater. Sci. Technol. 31(12), 1408–1416 (2015)
R. Kartikasari, Effect of aluminum content on microstructure and corrosion behavior of as cast Fe–Al–C alloys lightweight steel. Int. J. Appl. Eng. Res. 9(13), 2241–2249 (2014)
S. Khaple, R.G. Baligidad, M. Sankar, V.V. Satya Prasad, Effect of melting process and aluminium content on microstructure and mechanical properties of Fe–Al alloy. ISIJ Int. 50(10), 1483–1487 (2010)
J. Herrmann, G. Inden, G. Sauthoff, Deformation behaviour of iron-rich iron aluminum alloys at low alloys at low temperatures. Acta Mater. 51, 2847–2857 (2003)
I. Zuazo, B. Hallstedt, B. Lindahl, M. Selleby, M. Soler, A. Etienne, A. Perlade, D. Hasenpouth, V. Massardier-jourdan, S. Cazottes, X. Kleber, Low-density steels: complex metallurgy for automotive applications. JOM 66(9), 1747–1758 (2014)
L. Bartlett, V.D. Aken, High manganese and aluminum steels for the military and transportation industry. JOM 66(91), 1770–1784 (2014)
A. Schneider, L. Falat, G. Sauthoff, G. Frommeyer, Constitution and microstructures of Fe–Al–M–C–(M = Ti, V, Nb, Ta) alloys with carbides and Laves-phase. Intermetallics 11, 443–450 (2003)
R.G. Baligidad, Effect of niobium on microstructure and mechanical properties of hot-rolled Fe–8.5wt%Al–0.1wt% C alloy. J. Mater. Sci. 39, 5599–5602 (2004)
R.G. Baligidad, Effect of niobium on microstructure and mechanical properties of high carbon Fe–10.5wt.%Al alloys. Mater. Sci. Eng. A 368, 131–138 (2004)
L. Falat, A. Schneider, G. Sauthoff, G. Frommeyer, Mechanical properties of Fe–Al–M–C (M = Ti, V, Nb, Ta) alloys with strengthening carbides and Laves phase. Intermetallics 13, 1256–1262 (2005)
T. Gladman, The Physical Metallurgy of Microalloyed Steels, 1st edn. (Maney Publishing, London, 1997), pp. 1–363
J.G. Speer, A.L. Araujo, D.K. Matlock, E. de Moor, Nb-microalloying in next-generation flat-rolled steels: an overview. Mater. Sci. Forum 879, 1834–1840 (2016)
A. Ray, Niobium microalloyed rail steels. Mater. Sci. Technol. 33(13), 1–17 (2017)
M.D. Hecht, B.A. Webler, Y.N. Picard, Effects of Nb modification and cooling rate on the microstructure in an ultrahigh carbon steel. Metall. Mater. Trans. A 49, 2161–2172 (2018)
S. Khaple, V.V. Satya Prasad, B.R. Golla, Microstructural characterisation of Ti containing Fe–7Al–0.35C based low density steel. Trans. Indian Inst. Met. 71(11), 2713–2716 (2018)
S.Y. Shin, H. Lee, S.Y. Han, C.-H. Seo, K. Choi, S. Lee, N.J. Kim, J.-H. Kwak, K.-G. Chi, Correlation of microstructure and cracking phenomenon occurring during hot rolling of lightweight steel plates. Metall. Mater. Trans. A 41, 138–148 (2010)
S.S. Sohn, B.-J. Lee, S. Lee, J.-H. Kwak, Effects of aluminum content on cracking phenomenon occurring during cold rolling of three ferrite-based lightweight steel. Acta Mater. 61, 5626–5635 (2013)
S.Y. Han, S.Y. Shin, S. Lee, N.J. Kim, J.-H. Kwak, K.-G. Chin, Effect of carbon content on cracking phenomenon occurring during cold rolling of three light-weight steel plates. Metall. Mater. Trans. A 42, 138–146 (2011)
M. Palm, G. Inden, Experimental determination of phase equilibria in the Fe–Al–C system. Intermetallics 3, 443–454 (1995)
V. Raghavan, Phase Diagrams of Ternary Iron Alloys. Part I (ASM, Metals Park, 1987)
A. Zargaran, H.S. Kim, J.H. Kwak, N.J. Kim, Effect of Nb and C additions on the microstructure and tensile properties of lightweight ferritic Fe–8Al–5Mn alloy. Scr. Mater. 89, 37–40 (2014)
A. Zargaran, H.S. Kim, J.H. Kwak, N.J. Kim, Effect of C content on the microstructure and tensile properties of lightweight ferritic Fe–8Al–5Mn–0.1Nb alloy. Met. Mater. Int. 21(1), 79–84 (2015)
A.J. Deardo, Niobium in modern steels. Int. Mater. Rev. 48(6), 371–402 (2003)
M.G. Akben, I. Weiss, J.J. Jones, Dynamic precipitation and solute hardening in a V microalloyed steel and two Nb steels containing high levels of Mn. Acta Metall. 29, 111–121 (1981)
V.K. Sikka, S. Viswanathan, S. Vyas, Acceptable aluminum additions for minimal environmental effect in iron aluminides, in High Temperature Ordered Intermetallic Alloys V, vol. 288, ed. by I. Baker, R. Darolia, J.D. Whittenberger, M.H. Yoo (Materials Research Society, Pittsburgh, 1993), pp. 971–976
J.H. Chen, R. Cao, Micromechanism of Cleavage Fracture of Metals: A Comprehensive Microphysical Model for Cleavage Cracking in Metals (Elsevier, Amsterdam, 2015)
M. Calcagnotto, D. Ponge, Y. Adachi, D. Raabe, Effect of grain refinement on strength and toughness in dual-phase steels, in Proceedings of the 2nd International Symposium on Steel Science (ISSS 2009), Oct 21–24, ed. by K. Higashida, N. Tsuji (The Iron and Steel Institute of Japan, Kyoto, Japan, 2009)
Acknowledgements
The authors are grateful to Defence Research and Development Organization, New Delhi, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khaple, S., Prakash, U., Golla, B.R. et al. Effect of Niobium Addition on Microstructure and Mechanical Properties of Fe–7Al–0.35C Low-Density Steel. Metallogr. Microstruct. Anal. 9, 127–139 (2020). https://doi.org/10.1007/s13632-020-00622-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13632-020-00622-9