Abstract
The in vitro antibacterial activity of a series of phosphanegold(I) dithiocarbamates, R3PAu[S2CN(iPr)CH2CH2OH] where R = Ph (2), Cy (3) and Et (4), against 25 strains of Gram-positive and Gram-negative bacteria were determined through the disk diffusion method, the determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) and by time-kill assay. Compounds 2 and 3 have been shown to be specifically active against the tested Gram-positive bacteria, with MIC values ranging from 7.81 to 125 μg/ml. Compound 4 has a broad-spectrum activity against 24 strains of Gram-positive and Gram-negative bacteria, with MIC values ranging from 0.98 to 1,000 μg/ml. Noteworthy was that 4, with a very low MIC value of 0.98 μg/ml, is particularly effective against methicillin-resistant Staphylococcus aureus (MRSA) and Bacillus sp., as effective as the standard antibiotic ciprofloxacin. In time-kill studies, the bacteriostatic and bactericidal activities of the tested compounds towards susceptible strains were similar to their characteristics determined by MBC/MIC ratios. In the time-kill assay, 2 and 3 showed only bactericidal activity towards the susceptible strains tested, whereas 4 revealed varying degrees of bactericidal and bacteriostatic activities, results indicating different antibacterial mechanisms are involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emergence of resistance in pathogenic bacteria to multiple antibacterial agents has become a significant public health issue as there are fewer, or even sometimes no, effective antibiotic treatments available for these infectious diseases [1]; also see recent commentaries on this issue [2, 3]. Apart from patients themselves, the threat of multidrug-resistant bacteria posed towards frontline health workers has also increased [4]. Due to their natural behaviour of rapid life cycle, fast reproduction and the ability to exchange genetic information with other strains of bacteria, the chances of bacteria to develop resistance to currently available antibiotics are fairly high [5]. Hence, over and above improving health care hygiene, there is a clear imperative to develop novel antimicrobial agents needed to meet the challenges posed by the rapid emergence of multidrug-resistant pathogens. As a case in point, over time, the original Gram-positive bacterium Staphylococcus aureus developed resistance towards a series of first-line, second-line and even third-line antibiotics [6] to evolve into methicillin-resistant S. aureus (MRSA). Now, as MRSA is able to resist the beta-lactam group of antibiotics, the treatment of this highly prevalent pathogen has become an urgent challenge. As a contribution to the development of new and effective antibacterial agents, herein, synthetic gold compounds derived from phosphanegold(I) dithiocarbamate (Fig. 1) are demonstrated to exhibit convincing effects against a broad range of pathogens, including the particularly virulent bacterium, MRSA.
Since earliest civilization, gold and its compounds have been utilized by medical practitioners to treat various health-related problems, and amongst these are bacterial infections [7–10]. Despite this, antibacterial studies on gold compounds are still relatively limited [10–15] and more often than not focussing on gold(I) rather on gold(III) compounds. The primary focus of these studies is usually upon the inherent interest in the chemistry and consequently the accompanying antimicrobial work is often limited to the measurement of minimum inhibitory concentration (MIC) and sometimes minimum bactericidal concentration (MBC). One limitation of these studies is the inability of the method(s) to determine the kinetics of interaction between the putative antibacterial agents and the bacteria under investigation. In the present study, the MIC and MBC scores of some phosphanegold(I) dithiocarbamates (Fig. 1) against a wide range of Gram-positive and Gram-negative bacteria are determined. This is augmented by an assessment of the killing kinetics determined by a time-kill assay, therefore enhancing the understanding of the pharmaco-dynamic relationships between the phosphanegold(I) dithiocarbamates and their effects on bacteria.
Reflecting the increasing interest in the antimicrobial activity of gold compounds, very recently, a comprehensive review of the developments in this field appeared [16]. While studies have been reported on phosphanegold(I) mono-functional thiolate compounds, none have yet appeared for bi-functional dithiolate analogues, such as dithiocarbamate. This is perhaps a little surprising owing to the substantial and ongoing efforts investigating the anti-tumour potential of gold dithiocarbamates. Thus, directly related to the compounds shown in Fig. 1, i.e. phosphanegold(I) dithiocarbamates, several studies focussing on cytotoxicity profiles and mechanisms of cell death have appeared [17–19]. Even more studies of gold(III) dithiocarbamates are available as these potent compounds exhibit in vivo potential, have limited nephrotoxicity and a different mechanism of action to the widely used anti-cancer drug cisplatin, (NH3)2PtCl2 [20–24]. Herein, we redress this shortcoming in the gold/antimicrobial literature by reporting the exciting antibacterial activity of 2–4, as outlined above.
Experimental
Chemistry
The R3PAu[S2CN(iPr)CH2CH2OH] compounds, where R = Ph (2), Cy (3) and Et (4), were prepared from the reaction of the respective R3PAuCl precursor with the Na[S2CN(iPr)CH2CH2OH] salt as described in the literature [19]. The compounds exhibited the reported spectroscopic attributes (IR, 1H and 13C{1H} NMR). High-energy absorptions (CHCl3 solution) ascribed to intraligand (IL) dithiocarbamate transitions [25] were noted in the UV–vis spectra run on an Agilent Cary 60 UV–vis spectrophotometer, see Supplementary Materials Table S1 for data. Photoluminescence (PL) measurements were performed on an Agilent Varian Cary Eclipse Fluorescence Spectrophotometer using a Xenon flash lamp as the excitation source at room temperature, also in CHCl3 solution; see Supplementary Materials Table S1 for details. In the case of 3 and 4, the powder X-ray diffraction patterns recorded on a PANalytical Empyrean XRD system with Cu-Κα1 radiation (λ = 1.54056 Å) in the 2θ range 5 to 40° with a step size of 0.026° were consistent with the simulated patterns calculated using the single crystal data using X’Pert HighScore Plus [26]; see Supplementary Materials Fig. S1.
Antibacterial activity assay
The disk diffusion method was applied to screen for antibacterial activity in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines. A total of 25 bacterial strains were included in this study, namely Aeromonas hydrophilla ATCC 35654, Acinetobacter baumannii ATCC 19606, Bacillus cereus ATCC 10876, Bacillus subtilis ATCC 6633, Citrobacter freundii ATCC 8090, Enterobacter cloacae ATCC 35030, Enterobacter aerogenes ATCC 13048, Enterococcus faecalis ATCC 29212, Enterococcus faecium ATCC 19434, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, Listeria monocytogenes ATCC 19117, Proteus mirabilis ATCC 25933, Proteus vulgaris ATCC 13315, Pseudomonas aeruginosa ATCC 27853, Salmonella paratyphi A ATCC 9150, Salmonella typhimurium ATCC 14028, Shigella flexneri ATCC 12022, Shigella sonnei ATCC 9290, S. aureus ATCC 25923, methicillin-resistant S. aureus (MRSA) ATCC 43300, Staphylococcus saprophyticus ATCC 15305, Stenotrophomonas maltophilia ATCC 13637, Streptococcus pyogenes ATCC 49399 and Vibrio parahaemolyticus ATCC 17802. All bacterial cultures were purchased from American Type Culture Collection (ATCC). The inoculum suspension of each bacterial strain was adjusted to 0.5 McFarland standard turbidity (corresponding to approximately 108 CFU/ml) by adding Mueller-Hinton broth. This suspension was then swabbed on the surface of Mueller-Hinton agar (MHA) plates using a sterile cotton swab. The test compounds were dissolved in DMSO to achieve a test concentration of 2 mg/ml. Sterile 6-mm filter paper discs were aseptically placed on MHA surfaces and 5 μl of the dissolved test compound was immediately added to the discs. Each plate contained one standard antibiotic paper disc, serving as the positive control, one disc served as negative control (5 μl broth) and one disc served as solvent control (5 μl DMSO). The plates were incubated at 37 °C for 24 h. Antibacterial activity was evaluated by measuring the diameter of inhibition zone against each bacterial strains. Each experiment was performed in duplicate.
Determination of minimum inhibitory concentration and minimum bactericidal concentration
A broth micro-dilution method was used to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values according to the CLSI guidelines. The test compounds were serially twofold diluted in DMSO to achieve the range of test concentrations of 2,000–0.06 μg/ml and then placed into each well of a 96-well microplate. An inoculum suspension with density of 105 CFU/ml exponentially growing bacterial cells was added into each well. The 96-well microplates were incubated at 37 °C for 24 h. All tests were performed in triplicate. Four controls comprising medium with standard antibiotic (positive control), medium with DMSO (solvent control), medium with inoculum bacterial cells (negative control) and medium with broth only (negative growth control) were included in each test. Bacterial growth was detected by adding 50 μl of a 0.2 mg/ml p-iodonitrotetrazolium violet (INT) indicator solution into each of the microplate wells and incubated at 37 °C for 30 min under aerobic agitation. Where bacterial growth was inhibited, the suspension in the well remained clear after incubation with INT. The INT will react in the presence of bacterial activity, as indicated by a change from clear to a red colour. The lowest concentration of the test compound which completely inhibited bacterial growth was considered as the MIC. After MIC determination, an aliquot of 100 μl from each well which showed no visible growth was spread onto MHA and further incubated at 37 °C for 30 min. The MBC was defined as the lowest concentration of the tested compound that produced a 99.9 % reduction in bacterial viable count on the MHA.
Time-kill assay
Time-kill assays were performed by the broth macro-dilution method in accordance with the CLSI guidelines. Inoculum suspensions with approximately 105 CFU/ml of exponentially growing bacterial cells were used in this study. The test compound was added to 10 ml of inoculum suspensions with final concentrations corresponding to ½x MIC, MIC and 2x MIC. A growth control comprising the bacterial strain without the test compound was included in each trial. The inoculum cultures were incubated at 37 °C on an orbital shaker at 200 rpm. Aliquots were removed from the inoculum culture after timed intervals of incubation (i.e. 0, 1, 2, 3 and 4 h, and 0, 4, 8 and 24 h), and serial tenfold dilutions were prepared in saline as needed. The numbers of viable cells were determined by the plate count technique which involved plating 25 μl of each dilution on a MHA plate [27]. All plates were incubated at 37 °C for 24 h. The experiments were performed in triplicate. Data were analysed as killing curves by plotting the log10 colony forming unit per millilitre (cfu/ml) versus time (hours), and the change in bacterial concentration was determined. The viable bacterial cell count for the time-kill end point determination, i.e. bactericidal activity, is defined as a reduction of ≥3 log10 cfu/ml relative to the initial inoculum, whereas bacteriostatic activity corresponds to <3 log10 cfu/ml decrease relative to the initial inoculum [28].
Results and discussion
Chemistry
The R3PAu[S2CN(iPr)CH2CH2OH] compounds, where R = Ph (2), Cy (3) and Et (4), and sodium dithiocarbamate salt (1), became available in an earlier study describing their cytotoxicity against MCF-7R breast cancer cells and their differing pathways of causing cell death, i.e. apoptopic for 2 and necrotic for 3 and 4 [19]; the salt was non-cytotoxic. The motivation for the present study arose from recent observations of remarkable antimicrobial activities, specifically against Gram-positive bacteria, exhibited by related phosphanegold(I) thiocarbamate compounds, i.e. Ph3PAu[SC(OR)=N(tol-p)], for R = Me, Et and iPr [29], and by the knowledge that other metal dithiocarbamates have been reported to exhibit antimicrobial activity [30–32].
Crystal structure analysis on 3 and 4 [19] proved linear P–Au–S coordination geometries with the second sulphur atom oriented towards gold as indicated in Fig. 1. While the structure of 2 remains unverified, literature precedents suggest a similar coordination arrangement [33, 34]. 1H NMR spectra run in DMSO solution after 24 h were unchanged compared with freshly prepared solutions proving the stability of 2–4 in the time-frame of the biological studies. The gold compounds are insoluble in water.
Anti-bacterial activity
The antibacterial properties of 2–4 along with the dithiocarbamate salt 1 were evaluated against both Gram-positive and Gram-negative bacteria using the Kirby-Bauer disk diffusion method. According to the results collected in Table 1, 2–4 were specifically effective against all tested Gram-positive bacteria but against not Gram-negative bacteria. By contrast, 4 was the most active compound with significant inhibitory activity towards all the tested Gram-positive and Gram-negative pathogens except P. aeruginosa. This finding indicates that 2 and 3 has a similar inhibitory mechanism of action towards Gram-positive bacteria only, whereas 4 possesses wider spectrum of inhibitory activity against both Gram-positive and Gram-negative bacteria that is similar to that exhibited by the standard antibiotic ciprofloxacin.
The antibacterial activity of 1–4 were quantitatively assessed by determining their minimum inhibitory concentration (MIC) values and the results are tabulated in Table 2; a lower MIC value indicates a better antimicrobial agent as less compound is required to inhibit growth of the bacteria. The MIC values of compounds 2–4 were in the range 0.98–2,000.00 μg/ml, whereas ciprofloxacin was active in the range of 0.06–125.00 μg/ml, tetracycline in the range of 1.95–250.00 μg/ml and chloramphenicol in the range of 62.50–250.00 μg/ml towards susceptible tested bacteria. Salt 1 exhibited low activity and only shows inhibition against B. subtilis, L. monocytogenes and S. pyogenes, with high MIC values of 2,000.00, 2,000.00 and 500.00 μg/ml, respectively. This result points to the importance of phosphine gold in imparting antibacterial activity.
Compounds 2 and 3 were effective in inhibiting the growth of all tested Gram-positive bacteria with MIC values in the range of 7.81–62.5 and 31.25–125.00 μg/ml, respectively. As shown in Table 2, the antibiotic ciprofloxacin used as standard drug was more potent than the tested compounds 2 and 3 against Gram-positive bacteria at low concentration (MIC = 0.98–125.00 μg/ml) with the exception against S. pyogenes where it was less active compared with 2. However, standard drugs tetracycline and chloramphenicol were less potent than 2 and 3 against Gram-positive bacteria at higher concentration (MIC = 125.00–250.00 μg/ml) with exception against B. subtilis and S. saprophyticus. Interestingly, 4 displayed excellent inhibitory activity towards Gram-positive bacteria, with lower MIC values in the range 0.98–3.91 μg/ml, compared with 2 and 3, and the standard drugs ciprofloxacin, tetracycline and chloramphenicol,. In addition, 4, with MIC values in the range of 15.63–1,000.00 μg/ml, also showed moderate antibacterial activity against Gram-negative bacteria. The standard drugs ciprofloxacin, tetracycline and chloramphenicol showed remarkable high activity against Gram-negative bacteria (MIC = 0.06–250.00 μg/ml) compared with 4 with the exception of chloramphenicol against P. vulgaris.
The most susceptible strains towards 4 were methicillin resistant S. aureus (hereafter, MRSA) and Bacillus spp. (MIC = 0.98 μg/ml), followed by Staphylococcus spp. (MIC = 1.95 μg/ml), L. monocytogenes (MIC = 1.95 μg/ml), S. pyogenes (MIC = 1.95 μg/ml) and Enterococcus spp. (MIC = 1.95-3.91 μg/ml). These observations suggest potential efficacy for treatment against a variety of ailments as the susceptible bacterial strains typically cause disease in humans. For example, Staphylococcus spp., Enterococcus spp. and Bacillus spp. cause urinary tract infection, nosocomial infection and bacteraemia [35–37], L. monocytogenes causes listeriosis and meningitis [38] and S. pyogenes causes bacteraemia and meningitis [39]. Furthermore, the MIC values obtained for 4 were relatively lower than the MIC values for the aforementioned Ph3PAu[SC(OR)=N(tol-p)], for R = Me, Et and iPr [29], series of compounds against B. cereus (MIC = 1–4 μg/ml), S. aureus (MIC = 37 μg/ml), E. faecalis (MIC = 4 μg/ml) and E. faecium (MIC = 37 μg/ml), and gold sulfanylcarboxylates [10] against S. aureus (MIC = 6.25–200.00 μg/ml) and B. subtilis (MIC = 6.25–200.00 μg/ml). In summary, the data indicates that 4, with its high antibacterial activity, could be further developed as an antimicrobial agent towards MRSA, Staphylococcus spp., Bacillus spp. and Enterococcus spp.
The bactericidal properties of 2–4 against susceptible strains (i.e. excluding P. aeruginosa) were analysed by the minimum bactericidal concentration (MBC) assay and summarized as MBC/MIC ratios in Table 2. An antimicrobial agent is considered bactericidal if the MBC is not more than fourfold higher than the MIC, i.e. MBC/MIC ≤ 4 [40]. Compounds 1–3 were shown to be bactericidal (MBC/MIC ≤ 2) towards the susceptible Gram-positive strains with exception of 3, against E. faecium, with the MBC being eightfold higher than the MIC indicating bacteriostatic character. For 4, bactericidal activity was observed positive against B. cereus, B. subtilis, L. monocytogenes, S. saprophyticus, S. pyogenes, A. hydrophilla, S. paratyphi A, S. flexneri, S. Sonnei, P. mirabilis and V. parahaemolyticus, whereas bacteriostatic activity on E. faecalis, E. faecium, MRSA, S. aureus, A. baumannii, C. freundii, E. aerogenes, E. cloacae, E. coli, K. pneumonia, S. typhimurium, S. maltophilia and P. vulgaris was indicated. These results suggest that the bacteriostatic and bactericidal activities of 2–4 are dependent on the bacterial strain. This behaviour is similar to standard antibiotic ciprofloxacin, which is classified primarily as a bactericidal drug, so that the MBC/MIC ≤ 4 might have been predicted. Ciprofloxacin has been shown to kill bacteria by binding their DNA Gyrase subunit which causes inhibition of bacteria DNA replication [41]. However, the MBC values of ciprofloxacin towards E. faecium (MBC/MIC = 8), E. aerogenes (32), S. paratyphi A (8), S. maltophilia (64) and P. mirabilis (8) were fourfold, or more, higher than the MIC indicating bacteriostatic activity. The present findings confirm the conclusions of earlier studies [42, 43], where it was shown that the antibacterial agent vancomycin is generally bactericidal against S. aureus and pneumococci, but bacteriostatic against enterococci.
Time-kill assays have been widely used for in vitro investigations of new antimicrobial agents as these provide descriptive (qualitative) information on the pharmacodynamics of antimicrobial agents [44]. In the present study, only gold compounds with high activity towards susceptible bacteria strains, i.e. with MIC < 100 μg/ml, were selected for time-kill studies. The kinetic interaction between susceptible bacteria and 2–4 were examined at concentrations of two times the MIC (2x MIC), MIC and one-half of the MIC (½x MIC).
The kill kinetic profiles of 2 and 3 (Figs. 2 and 3) displayed rapid bactericidal activity towards all susceptible strains, showing a ≥3log10 reduction in viable cell count relative to the initial inoculum at all tested concentrations after 1 h exposure (Table 3). As expected from the determined MBC/MIC ratios, the time-kill assays for 2 towards B. cereus, B. subtilis, E. faecalis, E. faecium, L. monocytogenes, MRSA, S. aureus, S. saprophyticus and S. pyogenes were consistent with bactericidal characteristic. A similar conclusion is apparent for 3 towards B. cereus, B. subtilis, L. monocytogenes, MRSA and S. pyogenes.
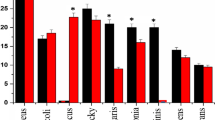
The kill kinetic profiles of 4, shown in Fig. 4, exhibited varying degrees of bactericidal and bacteriostatic activities depending on the tested strains and concentrations. After 1 h and at all concentrations tested, Table 3, 4 had a similar killing rate as 2 and 3 against B. cereus, B. subtilis and S. pyogenes. The killing rate of 4 was slower than 2 and 3 against E. faecalis, E. faecium, L. monocytogenes and MRSA in which bactericidal activities were only seen after 3 h interaction at 2x MIC. Compared to 2, 4 exhibited a slower killing rate against S. aureus and S. saprophyticus, showing bactericidal activity only after 3 h (2x MIC) and 4 h (½x MIC), respectively. In summary and consistent with the MBC/MIC ratios (Table 2), at its MIC value, 4 was found to be bactericidal towards B. cereus, B. subtilis, L. monocytogenes, S. saprophyticus and S. pyogenes after 24 h exposure. On the other hand, at its MIC value 4 is bacteriostatic towards E. faecalis, E. faecium, MRSA and S. aureus, as well as the Gram-negative bacteria E. coli and P. vulgaris.
Time-kill curves of 4 against a
B. cereus, b
B. subtilis, c
E. faecalis, d
E. faecium, e
L. monocytogenes, f
S. aureus (MRSA), g
S. aureus, h
S. saprophyticus, i
S. pyogenes, j
E. coli and k
P. vulgaris. The bactericidal level is indicated by the dashed lines
 , growth control
, growth control  , 2x MIC
, 2x MIC , MIC
, MIC  and ½x MIC
and ½x MIC 
Aggressive bactericidal activities for 4 can be achieved at concentrations higher than MIC, e.g. 2x MIC, over 24 h with E. faecalis, E. faecium, MRSA, S. aureus and E. coli. At its MIC value, 4 showed bacteriostatic activity against P. vulgaris (Fig. 4k) after 4 h contact but the strain regrew to the same level as the control inoculum after 24 h. Similarly, regrowth occurred in E. coli after 4 h of exposure to 4 at MIC and ½x MIC. This regrowth incidence was not found with the other strains tested, but is common in studies of bacterial killing rate with antimicrobial agents in time-kill assays [45]. The regrowth phenomenon was attributed to two distinct sub-populations with different susceptibility in which the selective growing of resistant sub-population take over the preferential killing of the susceptible sub-population at a specified time of interaction [44].
In order to place the time-kill assays determined for 2–4 in context, some observations from the literature are made. Vidaillac et al. [46] demonstrated that oritavancin exhibited rapid bactericidal activity against MRSA after 9 h exposure. In the present study, the kill kinetic profiles of 2 and 3 displayed much more rapid bactericidal activity, i.e. within 1 h, toward MRSA and other susceptible pathogens compared to oritavancin. Furthermore, the kill kinetic profiles of 4 exhibited both bactericidal and bacteriostatic activities depending on the tested strains and concentrations. The behaviour is similar to that exhibited by various oxazolidinone derivatives which demonstrated bacteriostatic effects toward Staphylococcus spp. and Enterococcus spp. but a bactericidal effect toward Streptococcus spp. [47].
In conclusion, three active gold compounds possess potent and differential activity against Gram-positive and Gram-negative bacteria pathogens, including the MRSA strain, which is often multi-resistant to several classes of antibiotics and can cause severe hospital-acquired and community-acquired infections. With rapid bactericidal activity against Gram-positive bacteria, 2 and 3 could provide clinical benefits over bacteriostatic therapy in neutropenia by rapid elimination of a bacterial pathogen and thereby reduce the likelihood of the spread of infection. With low MIC values, 4 could serve as a potential broad-spectrum antibacterial agent against Gram-positive and Gram-negative bacterial infections. The time-kill studies have provided valuable information on the rate, concentration and potential action of antibacterial agents in vitro. As the antibacterial activities and bacterial killing rates of 2–4 were different from each other, it is likely that different mechanisms are involved. Further investigation is needed to determine the mechanism(s) of action of these compounds in order to strengthen their potential as therapeutic antibiotics. Further derivatives, i.e. by varying both phosphane- and dithiocarbamate-substituents, will also be developed in a structure-activity study. In particular, of the present series, 4, with its potent and specific antibacterial profile, is deserving of further investigation and in vivo studies are planned.
References
Rai J, Randhawa GK, Kaur M (2013) Recent advances in antibacterial drugs. Int J Appl Basic Med Res 3:3–10. doi:10.4103/2229-516X.112229
McKenna M (2013) The last resort. Nature 499:394–396. doi:10.1038/499394a
Mole B (2013) Farming up trouble. Nature 499:398–400. doi:10.1038/499398a
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the infectious disease society of America. Clin Infect Dis 48:1–12. doi:10.1086/595011
D’Costa VM, King CE, Kalan L, Morar M, Sung WWL, Schwarz C, Froese D, Zazula G, Calmels F, Debruyne R, Golding GB, Poiner HN, Wright GD (2011) Antibiotic resistance is ancient. Nature 477:457–461. doi:10.1038/nature10388
Theuretzbacher U (2011) Resistance drives antibacterial drug development. Curr Opin Pharmacol 11:433–438. doi:10.1016/j.coph.2011.07.008
Kean WF, Kean IRL (2008) Clinical pharmacology of gold. Inflammopharmacology 16(3):112–125. doi:10.1007/s10787-007-0021-x
Pacheco EA, Tiekink ERT, Whitehouse MW (2009) Biomedical applications of gold and gold compounds. In: Corti C, Holliday R (eds) Gold: science and applications. CRC Press, Boca Raton, pp 217–230
Berners-Price SJ, Filipovska A (2011) Gold compounds as therapeutic agents for human diseases. Metallomics 3:863–873. doi:10.1039/c1mt00062d
Barreiro E, Cases JS, Couce MD, Sanchez A, Seoane R, Perez-Estevez A, Sordo J (2012) Synthesis and antibacterial activities of gold(I) sulfanylcarboxylates. Gold Bull 45:23–24. doi:10.1007/s13404-011-0040-7
Madeira JM, Gibson DL, Kean WF, Klegeris A (2012) The biological activity of auranofin: implications for novel treatment of diseases. Inflammopharmacology 20:297–306. doi:10.1007/s10787-012-0149-1
Noguchi R, Hara A, Sugie A, Nomiya K (2006) Synthesis of novel gold(I) complexes derived by AgCl-elimination between [AuCl(PPh3)] and silver(I) heterocyclic carboxylates, and their antimicrobial activities. Molecular structure of [Au(R, S-Hpyrrld)(PPh3)] (H2pyrrld = 2-pyrrolidone-5-carboxylic acid). Inorg Chem Commun 9:355–359. doi:10.1016/j.inoche.2006.01.001
Marques LL, Oliveira GMD, Lang ES, Campos MMAD, Gris LRS (2007) New gold(I) and silver(I) complexes of sulfamethoxazole: Synthesis, X-ray structural characterization and microbiological activities of triphenylphosphine(sulfamethoxazolato-N2)gold(I) and (sulfamethoxazolato)silver(I). Inorg Chem Commun 10:1083–1087. doi:10.1016/j.inoche.2007.06.005
Ray S, Mohan R, Singh JK, Samantaray MK, Shaikh MM, Panda D, Ghosh P (2007) Anticancer and antimicrobial metallopharmaceutical agents based on palladium, gold, and silver N-heterocyclic carbene complexes. J Am Chem Soc 129:15042–15053. doi:10.1021/ja075889z
Dinger MB, Henderson W (1998) Organogold(III) metallacyclic chemistry. Part 41. Synthesis, characterisation, and biological activity of gold(III)-thiosalicylate and -salicylate complexes. J Organomet Chem 560:233–243. doi:10.1016/S0022-328X(98)00493-8
Glišić BĐ, Djuran MI (2014) Gold complexes as antimicrobial agents: an overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. Dalton Trans 43:5950–5969. doi:10.1039/C4DT00022F
de Vos D, Ho SY, Tiekink ERT (2004) Cytotoxicity profiles for a series of triorganophosphinegold(I) dithiocarbamates and triorganophosphinegold(I) xanthates. Bioinorg Chem Appl 2:141–154. doi:10.1155/S156536330400010X
Keter FK, Guzei IA, Nell M, van Zyl WE, Darkwa J (2014) Phosphinogold(I) dithiocarbamate complexes: Effect of the nature of phosphine ligand on anticancer properties. Inorg Chem 53:2058–2067. doi:10.1021/ic4025926
Jamaludin NS, Goh Z-J, Cheah Y-K, Ang K-P, Sim J-H, Khoo CH, Fairuz ZA, Halim SNBA, Ng SW, Seng H-L, Tiekink ERT (2013) Phosphanegold(I) dithiocarbamates, R3PAu[SC(=S)N(iPr)CH2CH2OH] for R = Ph, Cy and Et: role of phosphane-bound R substituents upon in vitro cytotoxicity against MCF-7R breast cancer cells and cell death pathways. Eur J Med Chem 67:127–141. doi:10.1016/j.ejmech.2013.06.038
Ronconi L, Fregona D (2009) The midas touch in cancer chemotherapy: From platinum- to gold-dithiocarbamato complexes. Dalton Trans 10670–10680. doi:10.1039/B913597A
Casini A, Kelter G, Gabbiani C, Cinellu MA, Minghetti G, Fregona D, Fiebig H-H, Messori L (2009) Chemistry, antiproliferative properties, tumor selectivity, and molecular mechanisms of novel gold(III) compounds for cancer treatment: A systematic study. J Biol Inorg Chem 14:1139–1149. doi:10.1007/s00775-009-0558-9
Zhang X, Frezza M, Milacic V, Ronconi L, Fan Y, Bi C, Fregona D, Dou QP (2010) Inhibition of tumor proteasome activity by gold-dithiocarbamato complexes via both redoxdependent and -independent processes. J Cell Biochem 109:162–172. doi:10.1002/jcb.22394
Cattaruzza L, Fregona D, Mongiat M, Ronconi L, Fassina A, Colombatti A, Aldinucci D (2011) Antitumor activity of gold(III)-dithiocarbamato derivatives on prostate cancer cells and xenografts. Int J Can 128:206–215. doi:10.1002/ijc.25311
Nardon C, Schmitt SM, Yang H, Zuo J, Fregona D, Dou QP (2014) Gold(III) dithiocarbamate derivatives for the treatment of cancer: solution chemistry, DNA binding, and hemolytic properties. PLoS One 9:e84248. doi:10.1371/journal.pone.0084248
Tan YS, Sudlow AL, Molloy KC, Morishima Y, Fujisawa K, Jackson WJ, Henderson W, Halim SNBA, Ng SW, Tiekink ERT (2013) Supramolecular isomerism in a cadmium bis(N-hydroxyethyl, N-isopropyldithiocarbamate) compound: physiochemical characterization of ball (n = 2) and chain (n = ∞) forms of {Cd[S2CN(iPr)CH2CH2OH]2·solvent}n. Cryst Growth Des 13:3046–3056. doi:10.1021/cg400453x
2009 X’Pert HighScore Plus. PANalytical B.V., Almelo, The Netherlands
Cruishank R, Duguid JP, Marmion BP, Swain RHA (1975) The practice of medical microbiology. Churchill Livingstone, London
Petersen PJ, Jone CH, Bradford PA (2007) In vitro antibacterial activities of tigecycline and comparative agents by time-kill kinetic studies in fresh mueller-hinton broth. Diagn Microbiol Infect Dis 59(3):347–349. doi:10.1016/j.diagmicrobio.2007.05.013
Yeo CI, Sim J-H, Khoo C-H, Goh Z-J, Ang K-P, Cheah Y-K, Fairuz ZA, Halim SNBA, Ng SW, Seng H-L, Tiekink ERT (2013) Pathogenic Gram-positive bacteria are highly sensitive to triphenylphosphanegold(O-alkylthiocarbamates), Ph3PAu[SC(OR)=N(p-tolyl)] (R = Me, Et and iPr). Gold Bull 46:145–152. doi:10.1007/s13404-013-0091-z
Hogarth G (2012) Metal-dithiocarbamate complexes: chemistry and biological activity. Mini-Rev Med Chem 12:1202–1215. doi:10.2174/138955712802762095
Chauhan HPS, Bakshi A, Bhatiya S (2011) Synthetic, spectral as well as in vitro antimicrobial studies on some bismuth(III) bis(N, N-dialkyldithiocarbamato)alkylenedithiophosphates. Appl Organomet Chem 24:317–325. doi:10.1002/aoc.1609
Tiekink ERT (2008) Tin dithiocarbamates: applications and structures. Appl Organomet Chem 22:533–550. doi:10.1002/aoc.1441
Wijnhoven JG, Bosman WP, Beurskens PT (1972) Crystal and molecular structure of (triphenylphosphine)(N, N-diethyldithiocarbamato)gold(I). J Cryst Mol Struct 2:7–15. doi:10.1007/BF01198363
Ho SY, Tiekink ERT (2004) Crystal and molecular structures of two triorganophosphinegold(I) 2-amino-cyclopent-1-ene-1-carbodithioates. Z Kristallogr 219:513–518. doi:10.1524/zkri.219.8.513.38333
Baraboutis IG, Tsagalou EP, Lepinski JL, Papakonstantinou I, Papastamopoulos V, Skoutelis AT, Johnson S (2010) Primary Staphylococcus aureus urinary tract infection: the role of undetected hematogenous seeding of the urinary tract. Eur J Clin Microbiol Infect Dis 29:1095–1101. doi:10.1007/s10096-010-0967-2
Kau AL, Martin SM, Lyon W, Hayes E, Caparon MG, Hultgren SJ (2005) Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect Immun 74:2461–2468. doi:10.1128/IAI.73.4.2461-2468.2005
Bottone EJ (2010) Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382–398. doi:10.1128/CMR.00073-09
Schlech WF (2000) Foodborne Listeriosis. Clin Infect Dis 31:770–775. doi:10.1086/314008
Cunningham MW (2000) Pathogenesis of group A Streptococcal infections. Clin Microbiol Rev 13:470–511. doi:10.1128/CMR.13.3.470-511.2000
Levison ME (2004) Pharmacodynamics of antimicrobial drugs. Infect Dis Clin N Am 18:451–465. doi:10.1016/j.idc.2004.04.012
LeBel M (1988) Ciprofloxacin: chemistry, mechanism of action, resistance, antimicrobial spectrum, pharmacokinetics, clinical trials, and adverse reactions. Pharmacotherapy 8:3–30. doi:10.1002/j.1875-9114.1988.tb04058.x
Petersen PJ, Wang TZ, Dushin RG, Bradford PA (2004) Comparative in vitro activities of AC98-6446, a novel semisynthetic glycopeptide derivative of the natural product mannopeptimycin α and other antimicrobial agents against Gram-positive clinical isolates. Antimicrob Agents Chemother 48:739–746. doi:10.1128/AAC.48.3.739-746.2004
French GL (2006) Bactericidal agents in the treatment of MRSA infections-the potential role of daptomycin. J Antimicrob Chemother 58:1107–1117. doi:10.1093/jac/dkl393
Tam VH, Schilling AN, Nikolaou M (2005) Modelling time-kill studies to discern the pharmacodynamics of meropenem. J Antimicrob Chemother 55:699–706. doi:10.1093/jac/dki086
Belley A, Neesham-Grenon E, Arhin FF, McKay GA, Parr TR, Moeck G (2008) Assessment by time-kill methodology of the synergistic effects of oritavacin in combination with other antimicrobial agents against staphylococcus aureus. Antimicrob Agents Chemother 52:3820–3822. doi:10.1128/AAC.00361-08
Vidaillac C, Parra-Ruiz J, Rybak MJ (2011) In vitro time kill of oritavancin against clinical isolates of methicillin-resistant Staphylococcus aureus with reduced susceptibility to daptomycin. Diagn Microbiol Infect Dis 71:470–473. doi:10.1016/j.diagmicrobio.2011.09.002
Zurenko G, Yagi BH, Schaadt RD, Allison JW, Kilburn JO, Glickman SE, Hutchinson DK, Barbachyn MR, Brickner SJ (1996) In vitro of U-100592 and U-100766 novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother 40:839–845
Acknowledgments
The support from the Ministry of Higher Education, Malaysia, High-Impact Research scheme (UM.C/HIR-MOHE/SC/12) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 102 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sim, JH., Jamaludin, N.S., Khoo, CH. et al. In vitro antibacterial and time-kill evaluation of phosphanegold(I) dithiocarbamates, R3PAu[S2CN(iPr)CH2CH2OH] for R = Ph, Cy and Et, against a broad range of Gram-positive and Gram-negative bacteria. Gold Bull 47, 225–236 (2014). https://doi.org/10.1007/s13404-014-0144-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-014-0144-y




 , growth control
, growth control  , 2x MIC
, 2x MIC , MIC
, MIC  and ½x MIC
and ½x MIC 

 , growth control
, growth control  , 2x MIC
, 2x MIC  , MIC
, MIC  and ½x MIC
and ½x MIC 
