Abstract
Purpose
“Driver gene-negative” non-small cell lung cancer (NSCLC) currently has no approved targeted drug, due to the lack of common actionable driver molecules. Even though miRNAs play crucial roles in various malignancies, their roles in “driver gene-negative” NSCLC keep unclear.
Methods
miRNA expression microarrays were utilized to screen miRNAs associated with “driver gene-negative” NSCLC malignant progression. Quantitative real-time PCR (RT-qPCR) and in situ hybridization (ISH) were employed to validate the expression of miR-4739, and its correlation with clinicopathological characteristics was analyzed in tumor specimens using univariate and multivariate analyses. The biological functions and underlying mechanisms of miR-4739 were investigated both in vitro and in vivo.
Results
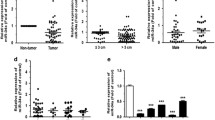
our research demonstrated, for the first time, that miR-4739 was substantially increased in “driver gene-negative” NSCLC tumor tissues and cell lines, and overexpression of miR-4739 was related to clinical staging, metastasis, and unfavorable outcomes. Functional experiments discovered that miR-4739 dramatically enhanced tumor cell proliferation, migration, and metastasis by promoting the epithelial-to-mesenchymal transition (EMT). Meanwhile, miR-4739 can be transported from cancer cells to the site of vascular epithelial cells through exosomes, consequently facilitating the proliferation and migration of vascular epithelial cells and inducing angiogenesis. Mechanistically, miR-4739 can activate Wnt/β-catenin signaling both in tumor cells and vascular epithelial cells by targeting Wnt/β-catenin signaling antagonists APC2 and DKK3, respectively.
Conclusion
Our work identifies a valuable oncogene, miR-4739, that accelerates malignant progression in “driver gene-negative” NSCLC and serves as a potential therapeutic target for this group of tumors.







Similar content being viewed by others
Data availability
All data sets generated during the study are available in the main text or supplementary materials. The raw data of miRNA and mRNA microarray were uploaded to the Gene Expression Omnibus (GEO) Database (GSE229301 and GSE229302, respectively). The data that support the findings of our study are available on request from the corresponding author (Neng Jiang).
Abbreviations
- EMT:
-
epithelial-mesenchymal transition
- NSCLC:
-
non-small cell lung cancer
- ICIs:
-
immune checkpoint inhibitors
- CTLA-4:
-
cytotoxic T-lymphocyte antigen 4
- PD-1:
-
programmed cell death protein 1
- PDL-1:
-
programmed cell death 1 ligand 1
- FBS:
-
fetal bovine serum
- NGS:
-
next-generation sequencing
- HUVECs:
-
human umbilical vein endothelial cells
- TVs:
-
tumor volumes
- SD:
-
standard deviation
- OS:
-
overall survival
- CCK8:
-
cell Counting Kit-8
- GSEA:
-
Gene Set Enrichment Analysis
- MVD:
-
microvessel density
- ISH:
-
in situ hybridization
- IHC:
-
immunohistochemistry
- IF:
-
immunofluorescence
- EdU:
-
5-Ethynyl-2′-deoxyuridine
- WB:
-
western blotting
- RT-qPCR:
-
reverse transcription‑quantitative PCR
References
N.S. Patil, B.Y. Nabet, S. Müller, H. Koeppen, W. Zou, J. Giltnane, A. Au-Yeung, S. Srivats, J.H. Cheng, C. Takahashi, P.E. de Almeida, A.S. Chitre, J.L. Grogan, L. Rangell, S. Jayakar, M. Peterson, A.W. Hsia, W.E. O’Gorman, M. Ballinger, R. Banchereau, D.S. Shames, Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. 40, 289–300e284 (2022)
S.R. Yang, A.M. Schultheis, H. Yu, D. Mandelker, M. Ladanyi, R. Büttner, Precision medicine in non-small cell lung cancer: current applications and future directions. Semin Cancer Biol. 84, 184–198 (2022)
D.S. Hong, M.G. Fakih, J.H. Strickler, J. Desai, G.A. Durm, G.I. Shapiro, G.S. Falchook, T.J. Price, A. Sacher, C.S. Denlinger, Y.J. Bang, G.K. Dy, J.C. Krauss, Y. Kuboki, J.C. Kuo, A.L. Coveler, K. Park, T.W. Kim, F. Barlesi, P.N. Munster, S.S. Ramalingam, T.F. Burns, F. Meric-Bernstam, H. Henary, J. Ngang, G. Ngarmchamnanrith, J. Kim, B.E. Houk, J. Canon, J.R. Lipford, G. Friberg, P. Lito, R. Govindan, B.T. Li, KRAS(G12C) inhibition with Sotorasib in Advanced Solid Tumors. N Engl. J. Med. 383, 1207–1217 (2020)
Y. Cui, W. Fang, C. Li, K. Tang, J. Zhang, Y. Lei, W. He, S. Peng, M. Kuang, H. Zhang, L. Chen, D. Xu, C. Tang, W. Zhang, Y. Zhu, W. Jiang, N. Jiang, Y. Sun, Y. Chen, H. Wang, Y. Lai, S. Li, Q. He, J. Zhou, Y. Zhang, M. Lin, H. Chen, C. Zhou, C. Wang, J. Wang, X. Zou, L. Wang, Z. Ke, Development and validation of a Novel signature to predict overall survival in “Driver Gene-negative” lung adenocarcinoma (LUAD): results of a Multicenter Study. Clin. Cancer Res. 25, 1546–1556 (2019)
Y. Chai, X. Wu, H. Bai, J. Duan, Combined immunotherapy with Chemotherapy versus Bevacizumab with Chemotherapy in First-Line treatment of driver-gene-negative non-squamous non-small cell Lung Cancer: an updated systematic review and network Meta-analysis, J. Clin. Med., 11, (2022)
W.J. Liu, Y. Du, R. Wen, M. Yang, J. Xu, Drug resistance to targeted therapeutic strategies in non-small cell lung cancer. Pharmacol. Ther. 206, 107438 (2020)
N.H. Hanna, B.J. Schneider, S. Temin, S. Jr. Baker, J. Brahmer, P.M. Ellis, L.E. Gaspar, R.Y. Haddad, P.J. Hesketh, D. Jain, I. Jaiyesimi, D.H. Johnson, N.B. Leighl, T. Phillips, G.J. Riely, A.G. Robinson, R. Rosell, J.H. Schiller, N. Singh, D.R. Spigel, J.O. Stabler, J. Tashbar, G. Masters, Therapy for Stage IV Non-Small-Cell Lung Cancer without driver alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 38, 1608–1632 (2020)
X. Si, R. Pan, S. Ma, L. Li, L. Liang, P. Zhang, Y. Chu, H. Wang, M. Wang, X. Zhang, L. Zhang, Genomic characteristics of driver genes in chinese patients with non-small cell lung cancer. Thorac. Cancer. 12, 357–363 (2021)
J. Mazieres, A. Drilon, A. Lusque, L. Mhanna, A.B. Cortot, L. Mezquita, A.A. Thai, C. Mascaux, S. Couraud, R. Veillon, M. Van den Heuvel, J. Neal, N. Peled, M. Früh, T.L. Ng, V. Gounant, S. Popat, J. Diebold, J. Sabari, V.W. Zhu, S.I. Rothschild, P. Bironzo, A. Martinez-Marti, A. Curioni-Fontecedro, R. Rosell, M. Lattuca-Truc, M. Wiesweg, B. Besse, B. Solomon, F. Barlesi, R.D. Schouten, H. Wakelee, D.R. Camidge, G. Zalcman, S. Novello, S.I. Ou, J. Milia, O. Gautschi, Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann. Oncol. 30, 1321–1328 (2019)
M. Russano, A. Cortellini, R. Giusti, A. Russo, F. Zoratto, F. Rastelli, A. Gelibter, R. Chiari, O. Nigro, M. De Tursi, S. Bracarda, S. Gori, F. Grossi, M. Bersanelli, L. Calvetti, V. Di Noia, M. Scartozzi, M. Di Maio, P. Bossi, A. Falcone, F. Citarella, F. Pantano, C. Ficorella, M. Filetti, V. Adamo, E. Veltri, F. Pergolesi, M.A. Occhipinti, L. Nicolardi, A. Tuzi, P. Di Marino, S. Macrini, A. Inno, M. Ghidini, S. Buti, G. Aprile, E. Lai, M. Audisio, S. Intagliata, R. Marconcini, D. Brocco, G. Porzio, M. Piras, E. Rijavec, F. Simionato, C. Natoli, M. Tiseo, B. Vincenzi, G. Tonini, D. Santini, Clinical outcomes of NSCLC patients experiencing early immune-related adverse events to PD-1/PD-L1 checkpoint inhibitors leading to treatment discontinuation. Cancer Immunol. Immunother. 71, 865–874 (2022)
W.Y. Xia, W. Feng, C.C. Zhang, Y.J. Shen, Q. Zhang, W. Yu, X.W. Cai, X.L. Fu, Radiotherapy for non-small cell lung cancer in the immunotherapy era: the opportunity and challenge-a narrative review. Transl Lung Cancer Res. 9, 2120–2136 (2020)
L. Khoja, D. Day, T. Wei-Wu, L.L. Chen, A.R. Siu, Hansen, Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann. Oncol. 28, 2377–2385 (2017)
G. Kara, G.A. Calin, B. Ozpolat, RNAi-based therapeutics and tumor targeted delivery in cancer. Adv. Drug Deliv Rev. 182, 114113 (2022)
N. Jiang, C. Zou, Y. Zhu, Y. Luo, L. Chen, Y. Lei, K. Tang, Y. Sun, W. Zhang, S. Li, Q. He, J. Zhou, Y. Chen, J. Luo, W. Jiang, Z. Ke, HIF-1ɑ-regulated miR-1275 maintains stem cell-like phenotypes and promotes the progression of LUAD by simultaneously activating Wnt/β-catenin and notch signaling. Theranostics. 10, 2553–2570 (2020)
A.A. Sayyed, P. Gondaliya, P. Bhat, M. Mali, N. Arya, A. Khairnar, K. Kalia, Role of miRNAs in Cancer Diagnostics and Therapy: a recent update. Curr. Pharm. Des. 28, 471–487 (2022)
L.F. Sempere, A.S. Azmi, A. Moore, microRNA-based diagnostic and therapeutic applications in cancer medicine. Wiley Interdiscip Rev RNA 12, e1662 (2021)
K. Yamana, J. Inoue, R. Yoshida, J. Sakata, H. Nakashima, H. Arita, S. Kawaguchi, S. Gohara, Y. Nagao, H. Takeshita, M. Maeshiro, R. Liu, Y. Matsuoka, M. Hirayama, K. Kawahara, M. Nagata, A. Hirosue, R. Toya, R. Murakami, Y. Kuwahara, M. Fukumoto, H. Nakayama, Extracellular vesicles derived from radioresistant oral squamous cell carcinoma cells contribute to the acquisition of radioresistance via the mir-503-3p-BAK axis. J. Extracell. Vesicles. 10, e12169 (2021)
L. Fang, J. Cai, B. Chen, S. Wu, R. Li, X. Xu, Y. Yang, H. Guan, X. Zhu, L. Zhang, J. Yuan, J. Wu, M. Li, Aberrantly expressed mir-582-3p maintains lung cancer stem cell-like traits by activating Wnt/β-catenin signalling. Nat. Commun. 6, 8640 (2015)
Y. Zhang, X. Wang, Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 13, 165 (2020)
A. Shams, R. Shabani, H. Asgari, M. Karimi, M. Najafi, M. Asghari-Jafarabadi, S.M. Razavi, S.R. Miri, M. Abbasi, A. Mohammadi, M. Koruji, In vitro elimination of EL4 cancer cells from spermatogonia stem cells by miRNA-143- and 206-loaded folic acid-conjugated PLGA nanoparticles. Nanomed. (Lond). 17, 531–545 (2022)
W. Du, R.E. Menjivar, K.L. Donahue, P. Kadiyala, A. Velez-Delgado, K.L. Brown, H.R. Watkoske, X. He, E.S. Carpenter, C.V. Angeles, Y. Zhang, M. Pasca di Magliano, WNT signaling in the tumor microenvironment promotes immunosuppression in murine pancreatic cancer, J. Exp. Med., 220 (2023)
J. Wang, H. Yu, W. Dong, C. Zhang, M. Hu, W. Ma, X. Jiang, H. Li, P. Yang, D. Xiang, M6A-mediated upregulation of FZD10 regulates liver cancer stem cells properties and lenvatinib resistance through WNT/β-catenin and Hippo signaling pathways, Gastroenterology, (2023)
M.J. Parsons, T. Tammela, L.E. Dow, WNT as a driver and dependency in Cancer. Cancer Discov. 11, 2413–2429 (2021)
F. Yu, C. Yu, F. Li, Y. Zuo, Y. Wang, L. Yao, C. Wu, C. Wang, L. Ye, Wnt/β-catenin signaling in cancers and targeted therapies. Signal. Transduct. Target. Ther. 6, 307 (2021)
H.B. Park, J.W. Kim, K.H. Baek, Regulation of wnt signaling through Ubiquitination and Deubiquitination in Cancers, Int. J. Mol. Sci., 21 (2020)
D.J. Stewart, Wnt signaling pathway in non-small cell lung cancer. J. Natl. Cancer Inst. 106, djt356 (2014)
Z. Wu, W. Shi, C. Jiang, Overexpressing circular RNA hsa_circ_0002052 impairs osteosarcoma progression via inhibiting Wnt/β-catenin pathway by regulating miR-1205/APC2 axis. Biochem. Biophys. Res. Commun. 502, 465–471 (2018)
W. Zhang, X. Ruan, Y. Li, J. Zhi, L. Hu, X. Hou, X. Shi, X. Wang, J. Wang, W. Ma, P. Gu, X. Zheng, M. Gao, KDM1A promotes thyroid cancer progression and maintains stemness through the Wnt/β-catenin signaling pathway. Theranostics. 12, 1500–1517 (2022)
F. Yang, H. Xiong, L. Duan, Q. Li, X. Li, Y. Zhou, MiR-1246 promotes Metastasis and Invasion of A549 cells by targeting GSK-3β–Mediated Wnt/β-Catenin pathway. Cancer Res. Treat. 51, 1420–1429 (2019)
C.L. Busceti, S. Marchitti, F. Bianchi, P. Di Pietro, B. Riozzi, R. Stanzione, M. Cannella, G. Battaglia, V. Bruno, M. Volpe, F. Fornai, F. Nicoletti, S. Rubattu, Dickkopf-3 upregulates VEGF in cultured human endothelial cells by activating activin receptor-like kinase 1 (ALK1) pathway. Front. Pharmacol. 8, 111 (2017)
M.H. Soheilifar, M. Pornour, M. Saidijam, R. Najafi, F. Azizi Jalilian, H. Keshmiri Neghab, R. Amini, miR-1290 contributes to oncogenesis and angiogenesis via targeting of THBS1, DKK3 and, SCAI, Bioimpacts, 12, 349–358 (2022)
R. Kalluri, V.S. LeBleu, The biology, function, and biomedical applications of exosomes, Science, 367 (2020)
W. Yu, J. Hurley, D. Roberts, S.K. Chakrabortty, D. Enderle, M. Noerholm, X.O. Breakefield, J.K. Skog, Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 32, 466–477 (2021)
Z. Zeng, Y. Li, Y. Pan, X. Lan, F. Song, J. Sun, K. Zhou, X. Liu, X. Ren, F. Wang, J. Hu, X. Zhu, W. Yang, W. Liao, G. Li, Y. Ding, L. Liang, Cancer-derived exosomal mir-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 9, 5395 (2018)
L. He, W. Zhu, Q. Chen, Y. Yuan, Y. Wang, J. Wang, X. Wu, Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 9, 8206–8220 (2019)
G. Kyriakopoulos, V. Katopodi, I. Skeparnias, E.G. Kaliatsi, K. Grafanaki, C. Stathopoulos, KRAS(G12C) can either promote or impair Cap-Dependent translation in two different lung Adenocarcinoma Cell Lines, Int. J. Mol. Sci., 22 (2021)
Y. Zeng, X. Yao, X. Liu, X. He, L. Li, X. Liu, Z. Yan, J. Wu, B.M. Fu, Anti-angiogenesis triggers exosomes release from endothelial cells to promote tumor vasculogenesis. J. Extracell. Vesicles. 8, 1629865 (2019)
Y. Zhang, L. Zhou, Y. Xu, J. Zhou, T. Jiang, J. Wang, C. Li, X. Sun, H. Song, J. Song, Targeting SMYD2 inhibits angiogenesis and increases the efficiency of apatinib by suppressing EGFL7 in colorectal cancer. Angiogenesis. 26, 1–18 (2023)
K. Han, F.W. Wang, C.H. Cao, H. Ling, J.W. Chen, R.X. Chen, Z.H. Feng, J. Luo, X.H. Jin, J.L. Duan, S.M. Li, N.F. Ma, J.P. Yun, X.Y. Guan, Z.Z. Pan, P. Lan, R.H. Xu, D. Xie, CircLONP2 enhances colorectal carcinoma invasion and metastasis through modulating the maturation and exosomal dissemination of microRNA-17. Mol. Cancer. 19, 60 (2020)
A.W. Lambert, R.A. Weinberg, Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer. 21, 325–338 (2021)
P. Liu, Z. Wang, X. Ou, P. Wu, Y. Zhang, S. Wu, X. Xiao, Y. Li, F. Ye, H. Tang, The FUS/circEZH2/KLF5/ feedback loop contributes to CXCR4-induced liver metastasis of breast cancer by enhancing epithelial-mesenchymal transition. Mol. Cancer. 21, 198 (2022)
K. Ono, C. Sogawa, H. Kawai, M.T. Tran, E.A. Taha, Y. Lu, M.W. Oo, Y. Okusha, H. Okamura, S. Ibaragi, M. Takigawa, K.I. Kozaki, H. Nagatsuka, A. Sasaki, K. Okamoto, S.K. Calderwood, T. Eguchi, Triple knockdown of CDC37, HSP90-alpha and HSP90-beta diminishes extracellular vesicles-driven malignancy events and macrophage M2 polarization in oral cancer. J. Extracell. Vesicles. 9, 1769373 (2020)
J.K. Min, H. Park, H.J. Choi, Y. Kim, B.J. Pyun, V. Agrawal, B.W. Song, J. Jeon, Y.S. Maeng, S.S. Rho, S. Shim, J.H. Chai, B.K. Koo, H.J. Hong, C.O. Yun, C. Choi, Y.M. Kim, K.C. Hwang, Y.G. Kwon, The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J. Clin. Invest. 121, 1882–1893 (2011)
L. Jiang, M. Yin, X. Wei, J. Liu, X. Wang, C. Niu, X. Kang, J. Xu, Z. Zhou, S. Sun, X. Wang, X. Zheng, S. Duan, K. Yao, R. Qian, N. Sun, A. Chen, R. Wang, J. Zhang, S. Chen, D. Meng, Bach1 represses Wnt/β-Catenin signaling and angiogenesis. Circ. Res. 117, 364–375 (2015)
P.P. Wong, J.M. Muñoz-Félix, M. Hijazi, H. Kim, S.D. Robinson, B. De Luxán-Delgado, I. Rodríguez-Hernández, O. Maiques, Y.M. Meng, Q. Meng, N. Bodrug, M.S. Dukinfield, L.E. Reynolds, G. Elia, A. Clear, C. Harwood, Y. Wang, J.J. Campbell, R. Singh, P. Zhang, T.J. Schall, K.P. Matchett, N.C. Henderson, P.W. Szlosarek, S.A. Dreger, S. Smith, J.L. Jones, J.G. Gribben, P.R. Cutillas, P. Meier, V. Sanz-Moreno, K.M. Hodivala-Dilke, Cancer Burden is controlled by Mural Cell-β3-Integrin regulated crosstalk with Tumor cells, cell, 181, 1346–1363e1321 (2020)
Y. Jia, T. Qin, X. Zhang, S. Liu, Z. Liu, C. Zhang, J. Wang, K. Li, Effect of bevacizumab on the tight junction proteins of vascular endothelial cells. Am. J. Transl Res. 11, 5546–5559 (2019)
H. Wang, Q. Deng, Z. Lv, Y. Ling, X. Hou, Z. Chen, X. Dinglin, S. Ma, D. Li, Y. Wu, Y. Peng, H. Huang, L. Chen, N6-methyladenosine induced mir-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol. Cancer. 18, 181 (2019)
L. Liu, M. Gu, J. Ma, Y. Wang, M. Li, H. Wang, X. Yin, X. Li, CircGPR137B/miR-4739/FTO feedback loop suppresses tumorigenesis and metastasis of hepatocellular carcinoma. Mol. Cancer. 21, 149 (2022)
X. Wang, Q. Chen, X. Wang, W. Li, G. Yu, Z. Zhu, W. Zhang, ZEB1 activated-VPS9D1-AS1 promotes the tumorigenesis and progression of prostate cancer by sponging miR-4739 to upregulate MEF2D. Biomed. Pharmacother. 122, 109557 (2020)
K. Wu, Z. Liu, C. Dong, S. Gu, L. Li, W. Wang, Y. Zhou, MiR-4739 inhibits the malignant behavior of esophageal squamous cell carcinoma cells via the homeobox C10/vascular endothelial growth factor A/phosphatidylinositol 3-kinase/AKT pathway. Bioengineered. 13, 14066–14079 (2022)
C.X. Liu, X. Li, F. Nan, S. Jiang, X. Gao, S.K. Guo, W. Xue, Y. Cui, K. Dong, H. Ding, B. Qu, Z. Zhou, N. Shen, L. Yang, L.L. Chen, Structure and degradation of circular RNAs regulate PKR activation in Innate Immunity. Cell. 177, 865–880e821 (2019)
M. Correia de Sousa, M. Gjorgjieva, D. Dolicka, C. Sobolewski, M. Foti, Deciphering miRNAs’ Action through miRNA Editing, Int. J. Mol. Sci., 20 (2019)
S. Zhao, Y. Mi, B. Zheng, P. Wei, Y. Gu, Z. Zhang, Y. Xu, S. Cai, X. Li, D. Li, Highly-metastatic colorectal cancer cell released miR-181a-5p-rich extracellular vesicles promote liver metastasis by activating hepatic stellate cells and remodelling the tumour microenvironment. J. Extracell. Vesicles. 11, e12186 (2022)
M. Qiao, T. Jiang, X. Liu, S. Mao, F. Zhou, X. Li, C. Zhao, X. Chen, C. Su, S. Ren, C. Zhou, Immune checkpoint inhibitors in EGFR-Mutated NSCLC: Dusk or Dawn? J. Thorac. Oncol. 16, 1267–1288 (2021)
E.D. Agerschou, P. Flagmeier, T. Saridaki, C. Galvagnion, D. Komnig, L. Heid, V. Prasad, H. Shaykhalishahi, D. Willbold, C.M. Dobson, A. Voigt, B. Falkenburger, W. Hoyer, A.K. Buell, An engineered monomer binding-protein for α-synuclein efficiently inhibits the proliferation of amyloid fibrils, Elife, 8 (2019)
V.S. LeBleu, J.P. Thiery, The Continuing search for causality between epithelial-to-mesenchymal transition and the metastatic fitness of Carcinoma cells. Cancer Res. 82, 1467–1469 (2022)
N. Mohammadi Ghahhari, M.K. Sznurkowska, N. Hulo, L. Bernasconi, N. Aceto, D. Picard, Cooperative interaction between ERα and the EMT-inducer ZEB1 reprograms breast cancer cells for bone metastasis. Nat. Commun. 13, 2104 (2022)
Y.D. Zhao, M. Muhetaerjiang, H.W. An, X. Fang, Y. Zhao, H. Wang, Nanomedicine enables spatiotemporally regulating macrophage-based cancer immunotherapy. Biomaterials. 268, 120552 (2021)
C.S. Blaha, G. Ramakrishnan, S.M. Jeon, V. Nogueira, H. Rho, S. Kang, P. Bhaskar, A.R. Terry, A.F. Aissa, M.V. Frolov, K.C. Patra, R. Brooks Robey, N. Hay, A non-catalytic scaffolding activity of hexokinase 2 contributes to EMT and metastasis. Nat. Commun. 13, 899 (2022)
J. An, Y. Du, X. Fan, Y. Wang, C. Ivan, X.G. Zhang, A.K. Sood, Z. An, N. Zhang, EGFL6 promotes breast cancer by simultaneously enhancing cancer cell metastasis and stimulating tumor angiogenesis. Oncogene. 38, 2123–2134 (2019)
X. Liu, S. Tan, H. Liu, J. Jiang, X. Wang, L. Li, B. Wu, Hepatocyte-derived MASP1-enriched small extracellular vesicles activate HSCs to promote liver fibrosis, Hepatology, (2022)
A.J. Cooper, L.V. Sequist, J.J. Lin, Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 19, 499–514 (2022)
K. Tang, N. Jiang, Y. Kuang, Q. He, S. Li, J. Luo, W. Jiang, Y. Chen, Y. Sun, L. Chen, Y. Chen, J. Zhu, Y. Cui, H. Wan, Z. Ke, Overcoming T790M mutant small cell lung cancer with the third-generation EGFR-TKI osimertinib. Thorac. Cancer. 10, 359–364 (2019)
Acknowledgements
We want to thank the State Key Laboratory of Oncology in South China (Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China) for providing guidance and technical assistance in the study.
Funding
This research was supported by the National Nature Science Foundation of China (Nos. 82103271) and the Guangdong Basic and Applied Basic Research Foundation (Nos. 2023A04J2126).
Author information
Authors and Affiliations
Contributions
N.J. and Y. L. designed the manuscript, analyzed the data, and wrote the manuscript. Y.L., W.C., W.Z., Q.Y., M.M., Y.S., and Q.H. collected patient samples, performed experiments, and analyzed the data. N.J. provided financial support. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All research adhered to the tenets of the Declaration of Helsinki. The Ethics Committee of Sun Yat-Sen University Cancer Center approved this study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenjian Cen, Qin Yan and Wenpeng Zhou have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cen, W., Yan, Q., Zhou, W. et al. miR-4739 promotes epithelial-mesenchymal transition and angiogenesis in “driver gene-negative” non-small cell lung cancer via activating the Wnt/β-catenin signaling. Cell Oncol. 46, 1821–1835 (2023). https://doi.org/10.1007/s13402-023-00848-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00848-z