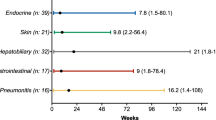
Abstract
Background
The prognostic relevance of early immune-related adverse events (irAEs) in patients affected by non-small cell lung cancer (NSCLC) upon immunotherapy is not fully understood.
Methods
The Leading to Treatment Discontinuation cohort included 24 patients experiencing severe irAEs after one of two administrations of single anti-PD-1/PD-L1 in any line setting for metastatic NSCLC between November 2015 and June 2019. The control cohort was composed of 526 patients treated with single anti-PD-1/PD-L1 in any line setting with no severe irAE reported. The primary end points were median progression-free survival, overall survival, objective response rate, risk of progression of disease and risk of death. The correlation of clinic pathological features with early severe irAEs represented the secondary end point.
Results
Median PFS was 9.3 and 8.4 months, median OS was 12.0 months and 14.2 months at a median follow-up of 18.1 and 22.6 months in the LTD cohort and in the control cohort, respectively. The ORR was 40% (95% CI 17.2–78.8) in the LTD cohort and 32.7% (95% CI 27.8–38.2) in the control cohort. The risk of disease progression was higher in the LTD cohort (HR 2.52 [95% 1.10–5.78], P = .0288).
Conclusions
We found no survival benefit in LTD cohort compared to the control cohort. However, early and severe irAEs might underly an immune anti-tumor activation. We identified a significant association with first-line immune checkpoints inhibitors treatment and good PS. Further studies on risk prediction and management of serious and early irAEs in NSCLC patients are needed.


Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CTCAE:
-
Common Toxicity Criteria for Adverse Events
- CI:
-
Confidence intervals (CI)
- ECOG-PS:
-
Eastern Cooperative Oncology Group-Performance Status
- HR:
-
Hazard ratios
- ICIs:
-
Immune checkpoints inhibitors
- irAEs:
-
Immune-related adverse events
- LTD cohort:
-
Leading to treatment discontinuation cohort
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Objective response rate
- OR:
-
Odds radios
- OS:
-
Overall survival
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
- PFS:
-
Progression-free survival
References
Ackermann CJ, Adderley H, Ortega-Franco A, Khan A, Reck M, Califano R (2020) First-line immune checkpoint inhibition for advanced non-small-cell lung cancer: state of the art and future directions. Drugs 80(17):1783–1797. https://doi.org/10.1007/s40265-020-01409-6
Berghmans T, Dingemans AM, Hendriks LEL, Cadranel J (2020) Immunotherapy for nonsmall cell lung cancer: a new therapeutic algorithm. Eur Respir J 55(2):1901907. https://doi.org/10.1183/13993003.01907-2019
Pabani A, Butts CA (2018) Current landscape of immunotherapy for the treatment of metastatic non-small-cell lung cancer. Curr Oncol 25(Suppl 1):S94–S102. https://doi.org/10.3747/co.25.3750
Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB (2017) Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 8:49. https://doi.org/10.3389/fphar.2017.00049 [published correction appears in Front Pharmacol 2017 8:311. https://doi.org/10.3389/fphar.2017.00311]
Baxi S, Yang A, Gennarelli RL et al (2018) Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 360:k793. https://doi.org/10.1136/bmj.k793
Ramos-Casals M, Brahmer JR, Callahan MK et al (2020) Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 6(1):38. https://doi.org/10.1038/s41572-020-0160-6
Martins F, Sofiya L, Sykiotis GP et al (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 16(9):563–580. https://doi.org/10.1038/s41571-019-0218-0
Haanen JBAG, Carbonnel F, Robert C et al (2018) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29(Suppl 4):iv264–iv266. https://doi.org/10.1093/annonc/mdy162
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168. https://doi.org/10.1056/NEJMra1703481
Das S, Johnson DB (2019) Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 7(1):306. https://doi.org/10.1186/s40425-019-0805-8
Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F (2020) Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 18(1):87. https://doi.org/10.1186/s12916-020-01549-2
Rogado J, Sánchez-Torres JM, Romero-Laorden N et al (2019) Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer 109:21–27. https://doi.org/10.1016/j.ejca.2018.10.014
Eggermont AMM, Kicinski M, Blank CU et al (2020) Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol 6(4):519–527. https://doi.org/10.1001/jamaoncol.2019.5570
Cortellini A, Bersanelli M, Buti S et al (2019) A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer 7(1):57. https://doi.org/10.1186/s40425-019-0527-y
Cortellini A, Bersanelli M, Santini D et al (2020) Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: a multicentre analysis of immune-related adverse events. Eur J Cancer 128:17–26. https://doi.org/10.1016/j.ejca.2019.12.031
Cortellini A, Buti S, Bersanelli M et al (2019) Evaluating the role of FAMIly history of cancer and diagnosis of multiple neoplasms in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: the multicenter FAMI-L1 study. Oncoimmunology 9(1):1710389. https://doi.org/10.1080/2162402X.2019.1710389
Cortellini A, Vitale MG, De Galitiis F et al (2019) Early fatigue in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: an insight from clinical practice. J Transl Med 17(1):376. https://doi.org/10.1186/s12967-019-02132-x
Cortellini A, Buti S, Santini D et al (2019) Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. Oncologist 24(6):e327–e337. https://doi.org/10.1634/theoncologist.2018-0618
Cortellini A, Chiari R, Ricciuti B et al (2019) Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer 20(4):237-247.e1. https://doi.org/10.1016/j.cllc.2019.02.006
Cortellini A, Tucci M, Adamo V et al (2020) Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer 8(2):e001361. https://doi.org/10.1136/jitc-2020-001361
Buti S, Bersanelli M, Perrone F et al (2021) Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer 142:18–28. https://doi.org/10.1016/j.ejca.2020.09.033
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Gridelli C, Balducci L, Ciardiello F et al (2015) Treatment of elderly patients with non-small-cell lung cancer: results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer 16(5):325–333. https://doi.org/10.1016/j.cllc.2015.02.006
Austin PC (2011) Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 10(2):150–161. https://doi.org/10.1002/pst.433
Sato K, Akamatsu H, Murakami E et al (2018) Correlation between immune-related adverse events and efficacy in Non-Small Cell Lung Cancer treated with nivolumab. Lung Cancer 115:71–74. https://doi.org/10.1016/j.lungcan.2017.11.019 [published correction appears in Lung Cancer 2018 126:230–231. https://doi.org/10.1016/j.lungcan.2018.11.007]
Akamatsu H, Murakami E, Oyanagi J et al (2020) Immune-related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced Non-Small Cell Lung Cancer. Oncologist 25(4):e679–e683. https://doi.org/10.1634/theoncologist.2019-0299
Grangeon M, Tomasini P, Chaleat S et al (2019) Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in Non-Small-Cell Lung Cancer. Clin Lung Cancer 20(3):201–207. https://doi.org/10.1016/j.cllc.2018.10.002
Ricciuti B, Genova C, De Giglio A et al (2019) Impact of immune-related adverse events on survival in patients with advanced Non-Small Cell Lung Cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 145(2):479–485. https://doi.org/10.1007/s00432-018-2805-3
Cortellini A, Friedlaender A, Banna GL et al (2020) Immune-related adverse events of pembrolizumab in a large real-world cohort of patients with NSCLC with a PD-L1 expression ≥ 50% and their relationship with clinical outcomes. Clin Lung Cancer 21(6):498-508.e2. https://doi.org/10.1016/j.cllc.2020.06.010
Berner F, Bomze D, Diem S et al (2019) Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in Non-Small Cell Lung Cancer. JAMA Oncol 5(7):1043–1047. https://doi.org/10.1001/jamaoncol.2019.0402 [published correction appears in JAMA Oncol 2019 5(7):1070. https://doi.org/10.1001/jamaoncol.2019.2137]
Weinmann SC, Pisetsky DS (2019) Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford) 58(Suppl 7):vii59–vii67. https://doi.org/10.1093/rheumatology/kez308
Teraoka S, Fujimoto D, Morimoto T et al (2017) Early immune-related adverse events and association with outcome in advanced Non-Small Cell Lung Cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol 12(12):1798–1805. https://doi.org/10.1016/j.jtho.2017.08.022
Hosoya K, Fujimoto D, Morimoto T et al (2020) Association between early immune-related adverse events and clinical outcomes in patients with Non-Small Cell Lung Cancer treated with immune checkpoint inhibitors. Clin Lung Cancer 21(4):e315–e328. https://doi.org/10.1016/j.cllc.2020.01.003
Wang Y, Zhou S, Yang F et al (2019) Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol 5(7):1008–1019. https://doi.org/10.1001/jamaoncol.2019.0393
Fukihara J, Sakamoto K, Koyama J et al (2019) Prognostic impact and risk factors of immune-related pneumonitis in patients with Non-Small-Cell Lung Cancer who received programmed death 1 inhibitors. Clin Lung Cancer 20(6):442-450.e4. https://doi.org/10.1016/j.cllc.2019.07.006
Jia XH, Geng LY, Jiang PP et al (2020) The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. J Exp Clin Cancer Res 39(1):284. https://doi.org/10.1186/s13046-020-01749-x
Lim SY, Lee JH, Gide TN et al (2019) Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res 25(5):1557–1563. https://doi.org/10.1158/1078-0432.CCR-18-2795
Fujimura T, Sato Y, Tanita K et al (2018) Serum levels of soluble CD163 and CXCL5 may be predictive markers for immune-related adverse events in patients with advanced melanoma treated with nivolumab: a pilot study. Oncotarget 9(21):15542–15551. https://doi.org/10.18632/oncotarget.24509
Jing Y, Liu J, Ye Y et al (2020) Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat Commun 11(1):4946. https://doi.org/10.1038/s41467-020-18742-9
Colen RR, Fujii T, Bilen MA et al (2018) Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Invest New Drugs 36(4):601–607. https://doi.org/10.1007/s10637-017-0524-2
Sakata Y, Kawamura K, Ichikado K et al (2019) The association between tumor burden and severe immune-related adverse events in Non-Small Cell Lung Cancer patients responding to immune-checkpoint inhibitor treatment. Lung Cancer 130:159–161. https://doi.org/10.1016/j.lungcan.2019.02.011
Suazo-Zepeda E, Bokern M, Vinke PC, Hiltermann TJN, de Bock GH, Sidorenkov G (2021) Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-021-02996-3
Santini FC, Rizvi H, Plodkowski AJ et al (2018) Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 6(9):1093–1099. https://doi.org/10.1158/2326-6066.CIR-17-0755
Kashiwabara K, Fujii S, Tsumura S, Sakamoto K (2020) Timing of resumption of immune checkpoint inhibitor therapy after successful control of immune-related adverse events in seven advanced Non-Small Cell Lung Cancer patients. Anticancer Drugs 31(8):872–875. https://doi.org/10.1097/CAD.0000000000000957
Fujita K, Uchida N, Kanai O, Okamura M, Nakatani K, Mio T (2018) Retreatment with pembrolizumab in advanced Non-Small Cell Lung Cancer patients previously treated with nivolumab: emerging reports of 12 cases. Cancer Chemother Pharmacol 81(6):1105–1109. https://doi.org/10.1007/s00280-018-3585-9
Mouri A, Kaira K, Yamaguchi O et al (2019) Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother Pharmacol 84(4):873–880. https://doi.org/10.1007/s00280-019-03926-y
Fujita K, Uchida N, Yamamoto Y et al (2019) Retreatment with anti-PD-L1 antibody in advanced Non-Small Cell Lung Cancer previously treated with anti-PD-1 antibodies. Anticancer Res 39(7):3917–3921. https://doi.org/10.21873/anticanres.13543
Haanen J, Ernstoff M, Wang Y et al (2020) Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J Immunother Cancer 8(1):e000604. https://doi.org/10.1136/jitc-2020-000604
Abou Alaiwi S, Xie W, Nassar AH et al (2020) Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J Immunother Cancer 8(1):e000144. https://doi.org/10.1136/jitc-2019-000144
Motzer RJ, Rini BI, McDermott DF et al (2019) Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 20(10):1370–1385. https://doi.org/10.1016/S1470-2045(19)30413-9 [published correction appears in Lancet Oncol 2019 20(10):e559. https://doi.org/10.1016/S1470-2045(19)30542-X] [published correction appears in Lancet Oncol 2020 21(6):e304. https://doi.org/10.1016/S1470-2045(20)30268-0] [published correction appears in Lancet Oncol 2020 21(11):e518. https://doi.org/10.1016/S1470-2045(20)30598-2]
Komiya K, Nakamura T, Abe T et al (2019) Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with Non-Small Cell Lung Cancer. Thorac Cancer 10(9):1798–1804. https://doi.org/10.1111/1759-7714.13149
Naqash AR, Ricciuti B, Owen DH et al (2020) Outcomes associated with immune-related adverse events in metastatic Non-Small Cell Lung Cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother 69(7):1177–1187. https://doi.org/10.1007/s00262-020-02536-5 [published correction appears in Cancer Immunol Immunother 2020 69(7):1189. https://doi.org/10.1007/s00262-020-02582-z]
Betof Warner A, Palmer JS, Shoushtari AN et al (2020) Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol 38(15):1655–1663. https://doi.org/10.1200/JCO.19.01464
Pedicord VA, Montalvo W, Leiner IM, Allison JP (2011) Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA 108(1):266–271. https://doi.org/10.1073/pnas.1016791108
Acknowledgements
The present manuscript is not under consideration elsewhere and it has not been published in any form or language. Its submission was approved by all the authors.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors were involved in the writing of this manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Alessio Cortellini received speaker fees and grant consultancies from Roche, MSD, BMS, AstraZeneca, Novartis, Astellas. Marco Russano received honoraria for consultancy and scientific talks from Roche, BMS, MSD, Boehringer Ingelheim, AstraZeneca. Daniele Santini received speaker fees and grant consultancies from Janssen, Astellas, BMS, MSD, Pfizer, Boeringher Ingelheim, Roche, Eisai, Incyte, Novartis. Marcello Tiseo received speakers’ and consultants’ fee from AstraZeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre. Marcello Tiseo received institutional research grants from AstraZeneca, Boehringer Ingelheim. Sebastiano Buti received honoraria as a speaker at scientific events and advisory role by Bristol-Myers Squibb (BMS), Pfizer; MSD, Ipsen, Roche, Eli-Lilly, AstraZeneca, Pierre Fabre and Novartis.
Ethics approval
The study was approved by the respective local ethical committees on human experimentation of each institution, after previous approval by the coordinating center. The procedures followed were in accordance with the precepts of Good Clinical Practice and the declaration of Helsinki. IRB reference for the control cohort: University of L’Aquila, Internal Review Board protocol number 32865, approved on July 24th, 2018. IRB reference for the LTD cohort: Campus Bio-medico University of Rome, protocol number 37/19 OSS approved on July 24th, 2019.
Consent to participate
All patients alive at the time of data collection provided an informed consent for the present retrospective analysis.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Russano, M., Cortellini, A., Giusti, R. et al. Clinical outcomes of NSCLC patients experiencing early immune-related adverse events to PD-1/PD-L1 checkpoint inhibitors leading to treatment discontinuation. Cancer Immunol Immunother 71, 865–874 (2022). https://doi.org/10.1007/s00262-021-03045-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03045-9