Abstract
The hyper-production of β-glucosidase by a local strain of Beauveria bassiana under submerged conditions is reported in this study. The initial screening of seven agricultural residues showed that the haulm of Bambara—an underutilized African legume—supported the highest β-glucosidase production; hence, statistical optimization of enzyme production was done using this biomass as the sole carbon source. Plackett–Burman design identified the concentrations of Bambara haulm, KCl, and NaCl as well as agitation speed and incubation time as the most significant factors affecting enzyme production. Subsequently, the central composite design predicted the optimal conditions (Bambara 57 g/L, KCl 302 mg/L, NaCl 154 mg/L, agitation speed 150 rpm, and incubation 223 h) for B. bassiana β-glucosidase production, which were further validated. The generated quadratic model was deemed significant judging from its F-value (201.63), adequate precision ratio (45.74), as well as the R2 (0.9988), adjusted R2 (0.9938), and predicted R2 (0.9195) values. The optimization resulted in a ~5.36-fold increase in enzyme levels from the unoptimized production of ~133 to 711 U/mL. The enzyme was also demonstrated to efficiently hydrolyze cellobiose, converting more than 90% of the substrate to glucose. These results further establish the resourcefulness of the B. bassiana strain for the production of β-glucosidase enzyme, having immense potential, especially in the food and energy industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Beta-glucosidases (E.C.3.2.1.21) or β-glucosidases or Bgls is a class of glycosyl hydrolases that catalyze the hydrolysis of β-glycosidic bonds in short-chain oligosaccharides and alkyl- and aryl β-D-glucosides. Bgls are essential components of the cellulase enzymatic system which also comprises the endoglucanases (E.C.3.2.1.4) and the exoglucanases (E.C.3.2.1.91), which all act in tandem to convert cellulosic material into glucose. However, each enzyme component plays a distinct role in the system, while endoglucanases target the internal areas of amorphous cellulose, exoglucanases act at the reducing and non-reducing ends of the polymer [1]. The terminal reaction is catalyzed by Bgls which hydrolyze the cellobiose (released by the action of endoglucanase and exoglucanases) into glucose monomers [2]. Therefore, the efficient hydrolysis of cellulose, especially for the production of biofuels, is facilitated by the enzymatic actions of Bgls. In addition, the enzymes also possess some side activities that have been explored for other industrial applications [3]. These activities include their trans-glycosylation potential which serves as the basis of their use in the pharmaceutical industry for oligosaccharide synthesis, hydrolysis of glycosides which is useful in the beverage industries for the improvement of aroma, and the production of alkyl glucosides that are useful in cosmetics and detergents production [4].
With the global shift towards cleaner energy production as highlighted in the UN Sustainable Developmental Goal (UN SDG) 7, Bgls have become prominent among the most sought-after enzymes. Consequently, Bgls were estimated to account for one-quarter of the global agricultural enzymes market, valued at ~USD 380 million in 2021 and projected to reach ~USD 760.23 million by 2027 [5]. Bgls from several fungal genera such as Aspergillus [6], Fusarium [7], and Trichoderma [8] have been demonstrated for their significant functional properties and their suitability for industrial usage. However, one of the major bottlenecks for the utilization of Bgls in the industry is their high production cost, which results in high overhead costs. Thus, the possible approaches to address this constraint include finding novel isolates capable of utilizing low-cost media for enzyme production, optimizing fermentation conditions for maximal enzyme release as well as genetic engineering of the strain for heightened Bgl productivity and improved functionality.
In the last five decades, various scientific attempts—including the recent ones—have been made at addressing the challenges mentioned above [9, 10, and 11]. For instance, various novel microbial strains have been demonstrated to utilize different readily available growth substrates for Bgl production, such as the fungi, Aspergillus tubingensis, and Trichoderma reesei which utilized maize bran [9] and wheat bran [12], respectively, as well as the actinomycetes Jiangella alba which utilized wheat straw for the same purpose [13]. Similarly, Bgl genes from numerous microbes have been successfully cloned and expressed in efficient host systems for increased enzyme yields and ease of purification. In the study by Senba et al. [10], Bgl gene from Aspergillus chevalieri MK86 was expressed in Aspergillus oryzae to achieve a 100-fold increase in production relative to the native organism. Similarly, a recombinant Bgl with a 9-fold increased production level was obtained when the gene from Devosia sp. Arc12 was expressed in Escherichia coli BL21 (DE3) [11]. However, like for all other industrially important enzymes, the search for the optimum microbial source that would overcome one or more of the current limitations hindering the significant utilization of Bgl in the industry continues [14].
Recently, a strain of the entomopathogenic fungal endophyte, Beauveria bassiana, was discovered as a novel source of industrially important enzymes. Typically, B. bassiana is used in the formulation of biopesticides; it was also highlighted for its ability to produce various bioactive compounds such as beauvericin, bassianin, beauveriolides, tennelin, and oosporein [15]. The fungus is non-pathogenic to humans, animals, and plants [16]; hence, it is considered safe for human use as well as for the environment. B. bassiana SAN01 was observed to proliferate rapidly on various lignocellulosic biomass while simultaneously secreting significant quantities of lignocellulosic degrading enzymes, making it a potential mining site for industrial carbohydrases [17]. It was further observed during preliminary screening that the fungus’ Bgl production level under unoptimized conditions was higher than the previously reported for any Beauveria strain or other entomopathogenic fungal endophyte.
Process parameter optimization has been considered critical in the quest for cost-effective and enhanced enzyme production as parameters such as temperature, pH, fermentation time, and media components profoundly affect enzyme production [18]. Historically, the optimization of fermentation processes entails the systematic alteration of an individual independent variable or factor, while maintaining the other variables at constant values, a process that is quite slow and labor-intensive, especially when dealing with a substantial number of independent variables [19]. However, the conventional optimization approach fails to account for the interplay between the variables utilized and their overall impact on the required output; hence, there is a growing preference for statistical techniques such as response surface methodology (RSM) in the optimization of fermentation processes [20]. This statistical approach decreases the overall number of required experiments and better elucidates the relationships between various elements and the fermentation output [21]. RSM includes Plackett–Burman design (PBD) which efficiently identifies the most influential fermentation components out of many and the central composite design (CCD) which ascertains the ideal concentration of individual components, evaluates their interaction, and also assesses their impact on the response [22].
Thus, B. bassiana SAN01, a lignocellulosic-degrading strain of B. bassiana, was investigated for its potential to serve as an industrial source of Bgl based on the safety profile of the entomopathogenic fungal endophyte, as well as its notable industrial importance. In this regard, the study was aimed at maximizing the production of Bgl by B. bassiana SAN01 using readily available agricultural residues under submerged fermentation. To achieve this, RSM at two levels was employed to optimize the fermentation parameter involved in B. bassiana Bgl production, followed by the validation of the derived model. In addition, the efficiency of the Bgl in cellobiose hydrolysis was also assessed in this study. To the best of our knowledge, this is the first attempt at the production of Bgl by any Beauveria strain using lignocellulosic biomass as the substrate and optimizing the process via a statistical approach.
2 Methodology
2.1 Materials
The lignocellulosic biomass used in this study was obtained locally in Durban, South Africa. Chemicals including 4-nitrophenyl β-D-glucopyranoside (pNPG), cellobiose, and glucose were purchased from Sigma-Aldrich (South Africa). All other reagents used in this study were of HPLC or analytical grade. The Design Expert 11.0 (Stat-Ease, Minneapolis, USA) software was used for both the first (Plackett–Burman design) and second (central composite design) level optimization as well as for the validation of the generated model.
2.2 B. bassiana SAN01 culture conditions
The endophytic fungus Beauveria bassiana (Gene Accession Number: MN544934) selected for the production of β-glucosidase was obtained from the culture collection of the Department of Biotechnology and Food Science, Durban University of Technology, Durban, South Africa. The fungus was grown on potato dextrose agar (PDA) at 30 °C for 5 days. Afterward, B. bassiana spore suspension (1 × 106 spores/mL) was prepared following the protocol of Amobonye et al. [23].
2.3 Screening of lignocellulosic biomass as the main carbon source
B. bassiana SAN01 Bgl production was evaluated using seven different biomass samples (aspen wood, Bambara haulm, corn cob, molasses bran, sugarcane bagasse, wheat bran, and wheat straw). Fermentation was carried out in 250 mL Erlenmeyer flasks with 100 mL mineral salt solution containing (g/L) (NH4)2SO4 (4.0), KH2PO4 (1.0), MgSO4.7H2O (0.5), KCl (0.5), NaCl (0.25), CaCl2 (0.2), FeSO4 (0.1), and 40 g/L of the respective biomass at pH 6.0. The flask was inoculated with 1 mL of spore suspension (1 × 106 spores/mL) and incubated for 8 days at 30 °C and 150 rpm. Afterward, the culture broth was filtered using a sterile muslin cloth and centrifuged for 15 min at 4 °C and 10,000 × g. The supernatant retrieved after centrifugation was used as the crude enzyme for subsequent experiments.
2.4 Enzyme assay
β-D-glucopyranoside (pNPG) was used as the chromogenic substrate for the quantification of B. bassiana SAN01 Bgl activity. The reaction mixture comprised 0.9 mL of pNPG (5 mM) and 0.1 mL of the appropriately diluted enzyme, incubated at 60 °C for 10 min. The reaction was terminated by the addition of 1 mL of Na2CO3 (0.5 M), and absorbance was read at 410 nm using a UV-Vis spectrophotometer (Shimadzu UV- 1900i). One unit of β-glucosidase activity was defined as the amount of enzyme that generated 1 µmol of p-nitrophenol in 1 min at 35 °C [24]. The protein content of the samples was also determined by a modified Lowry method using bovine serum albumin as the standard [25].
2.5 Statistical optimization of the fermentation conditions
2.5.1 Plackett–Burman design (PBD)
The screening of the fermentation media components to identify the ones with significant effects on the production level of B. bassiana SAN01 Bgl was conducted using PBD [26]. This approach employs a linear methodology for identifying the important components while disregarding any potential interactions among the components following the first-order polynomial as represented in Eq. (1).
Y is the mean of Bgl activity; β0 is the intercept of the model; βi is the linear coefficient; Xi is the the value of an independent component.
Optimization at this level involved a total of 11 components, consisting of nine production media components and two physical components, which were selected from preliminary studies and previous literature [27, 28]. The experimental design included 13 runs, with 1 center point. The analysis of each component within the medium was evaluated at high (+ 1) and low (−1) levels; the real and coded range of the investigated factors are summarized in Table 1. The experiments were conducted in triplicate, and the mean value of Bgl activity (U/mL) served as the measured response. The components in the production medium that exhibited confidence levels over 95% were deemed significant to Bgl production and were subsequently optimized in CCD.
2.6 Central composite design (CCD)
CCD was utilized to determine the optimal values of the selected factors obtained from PBD analysis viz., concentrations of the carbon source (Bambara haulm), KCl, and CaCl2 as well as the incubation time and agitation speed. The five selected variables were investigated at five coded levels (−β, −1, 0, 1, β ) with Table 2 summarizing their actual and coded range values. The remaining eight components were kept at values equivalent to the central point values employed in the PBD. All runs were conducted in triplicates and the response was the calculated average Bgl activity. The relationships among the variables were established via a second-order polynomial equation as represented in Eq. (2).
Y is the mean of Bgl activity; Xi and Xj are the independent components; β0 is the intercept constant, βi is the linear coefficients; βii is the quadratic coefficient; βij is the coefficient of two-component interaction. Each variable in the equation was fitted to determine its significance, and an ANOVA (analysis of variance) test was performed to observe the goodness of fit. The interactions among the evaluated components were represented and visualized in contour plots [29].
Furthermore, to validate the model and the optimal concentration of the variables (A, B, C, D, and E) required for maximal B. bassiana Bgl production, the experiments were performed with the values predicted by the model; finally, correlation analysis was performed on the predicted and obtained response values for each solution [30].
2.7 Hydrolysis of cellobiose by B. bassiana SAN01 β-glucosidase
B. bassiana SAN01 Bgl was partially purified and concentrated via (NH4)2SO4 precipitation [31] and utilized in the hydrolysis of cellobiose. The 20 mL reaction mixture contained cellobiose at a final concentration of 1700 µg/mL and 50 U/mL of the B. bassiana Bgl in 0.1 mM sodium acetate buffer (pH 5.0) [32]. The reaction was performed for 24 h at 35 °C, samples were withdrawn at intervals, and the reaction was terminated by heating for 5 min at 100 °C in a boiling water bath. The supernatant was collected by centrifugation for 10 min at 10,000 × g and subsequently subjected to thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) to confirm cellobiose hydrolysis. TLC was done on silica gel plates (60F254, Merck Co) using a solvent system made up of n-butanol, acetic acid, and water (2:1:1 v/v); the plates were dried for 5 min at 121 °C and developed using orcinol reagent prepared in 10% sulfuric acid [33]. Furthermore, the concentrations of unhydrolyzed cellobiose and that of the released glucose were examined via HPLC using an Aminex HPX-87 H column (BIO-RAD) ran with acetonitrile and water (1:2 v/v) at a flow rate of 0.7 mL/min. Elution was monitored using a RID detector (RID-10, Shimadzu).
2.8 Statistical analyses
Statistical variance (ANOVA) was calculated with GraphPad Prism software (version 10), and data were all presented as the mean ± standard deviation of triplicate values. Differences between samples were undertaken to be statistically significant if p < 0.05.
3 Results and discussion
3.1 Valorization of lignocellulosic biomass for β-glucosidase production
In this study, B. bassiana SAN01 was observed to proliferate remarkably on different lignocellulosic biomass and produced Bgl in high quantities. Amongst the biomass tested, Bambara haulm and wheat bran were the most promising, supporting higher β-glucosidase yields of 132.71 U/mL and 87.80 U/mL, respectively (Fig. 1). The significant production level of Bgl recorded with Bambara haulm in this study is the first report utilizing the agricultural residue for Bgl production. Bambara is one of the underutilized pulse crops in Africa; however, the residue after harvest (the haulm) is usually burnt on the farm or used as fodder for livestock animals [34]. However, recent studies have shown that it contains a significant quantity of cellulose (37%) and hemicellulose (15%) [35]; hence, it possesses the potential for valorization, which was successfully explored in this study for B. bassiana Bgl production. Although there is currently no information detailing the underlying factors behind the efficient utilization of Bambara haulm as a fermentation substrate, we hypothesize that being a leguminous plant, it contains an adequate amount of required nutrients to support robust fungal metabolism and enzyme production. Furthermore, as observed in wheat bran and other lignocellulosic biomass, its constituent biopolymers such as cellulose and hemicellulose can upregulate the expression of relevant genes, leading to significant enzyme production. Wheat bran has been the ideal carbon source for industrial biocatalysts such as microbial amylases, cellulases, and xylanases [36, 37]. Various studies have described the efficient utilization of wheat bran by fungi for Bgl production including the recent 812 U/mL attained by Aspergillus versicolor [38]. However, in this study, it stood as the second most important biomass for the Bgl production after Bambara. On the other hand, aspen wood shaving and sugarcane bagasse could not support B. bassiana SAN01 Bgl production despite their high carbon content. For example, sugarcane bagasse comprises 40–50% cellulose, 25–35% hemicellulose, and 5–15% lignin [39]; however, its low nitrogen content might be responsible for its inability to promote enzyme production in this study, as well as aspen wood shavings. Interestingly, it was remarkable to observe that the unoptimized Bgl production level obtained from B. bassiana SAN01 in this study is 25.38-fold higher than the 5.20 U/mL from another strain of Beauveria [40]. Bambara haulm, being the best biomass for Bgl production by B. bassiana SAN01, was further used for optimization using RSM.
3.2 Screening of significant variables by Plackett–Burman design
In this study, a 13-PBD run was utilized to screen the fermentation factors with significant effects on B. bassiana SAN01 Bgl production. The outcomes of the first level optimization are summarized in Table 3; the experiments showed that five factors possessed significant effects on enzyme production. In this regard, the statistical significance of these effects is illustrated on the Pareto chart (Fig. 2), which highlights the concentration of Bambara, KCl, and NaCl as well as the incubation time and agitation speed as the critically important parameters influencing the production of the Bgl. Similarly, coefficient estimates also demonstrate the correlation and weight of each highlighted factor to Bgl production (Supplementary data). Furthermore, production levels of between 157.14 and 527.86 U/mL were observed in real time, while the highest observed and predicted production levels were recorded to be 527.86 and 511.57 U/mL, respectively. The p value < 0.05 indicates the significance of the corresponding factors, with the incubation time, KCl, NaCl, Bambara concentration, and agitation speed with p values ranging from <0.0001 to 0.0062 were established as the most significant factors (Table 4). These results are in agreement with the previous studies that identified incubation time [19, 41] and agitation speed [38, 41] as significant factors in Bgl production by different microorganisms. However, a negative correlation between KCl concentration and enzyme production was observed in this study; this is in contrast to a study on Aspergillus versicolor, where the same salt displayed a positive relationship with Bgl production [38]. Furthermore, the F-value of 36.86 recorded in this study implies the model was significant, and there is only a 0.01% chance that the F-value could result from noise. The predicted R2 of this model was recorded to be 0.8792, which was in reasonable agreement with the adjusted R² of 0.9442 (Table 3). Thus, the first-order regression equation showed Bgl activity as a linear function of the concentrations of Bambara (A), KCl (D), and NaCl (F) as well as agitation speed (J) and incubation time (L).
Bgl production level = 292.19 + 101.72 A − 46.57D − 41.29 F + 39.92 J + 64.95 L.
Hence, the ANOVA analysis and regression coefficients all established that the predicted model for B. bassiana SAN01 Bgl production was sufficient to annotate the significance of the five chosen factors; thus, these factors were utilized subsequently in CCD for the optimization of the response and the curvature of B. bassiana Bgl yield.
3.3 Optimization by central composite design and model validation
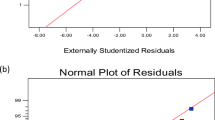
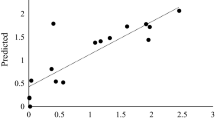
The optimal concentration of the significant factors obtained from PBD was determined using CCD for maximum Bgl production, the 23 full factorial design was also utilized to study the interactions between the selected factors. The 26-run experiments and the observed output are depicted in Table 4. In addition to the data presented in the table, the actual vs. predicted plots also elucidate the close agreement between the experimental and predicted output, further highlighting the significance of RSM in the optimization of fermentation conditions for enhanced B. bassiana SAN01 Bgl production (Fig. 3). The analysis of the coefficient estimates of the CCD revealed the positive linear effects of incubation time, Bambara concentration, and agitation speed and the negative effects of KCl and NaCl concentrations on Bgl production (Supplementary data). The observed experimental values vary from 258.7 to 711 U/mL (Table 5). ANOVA results suggested the adequacy of the second-order response; in this regard, the model F-value of 201.63 indicates the model’s significance. The goodness of fit of the model was illustrated by the reasonable agreement between the predicted R² (0.9195) and the adjusted R² (0.9938) (Table 6). Adeq Precision is a measure of the signal-to-noise ratio, with a value greater than 4 being desirable [22, 42]. Hence, the obtained Adeq Precision ratio of 45.74 in this study indicates an adequate signal; consequently, the model can be used to navigate the design space [22]. Furthermore, the factors both in their individual and interactive capacities—A, B, C, D, E, AB, BC, BE, CD, CE, DE, A², D², and E² were recorded with p values < 0.05; thus, they were all deemed to be significant and reliable.
The regression equation derived from the ANOVA demonstrated that Bgl production is a function of A is the concentration of Bambara, B is the KCl concentration, C is the NaCl concentration, D is the agitation speed, and E is the incubation time. Thus, the model quadratic regression is given as:
Bgl activity = 515.41 + 05.15 A -28.33 B − 32.41 C − 55.82 D + 20.53 E + 21.97 AB + 6.94 AC + 15.06 AD + 7.63 AE − 17.80 BC − 9.61 BD − 39.96 BE − 25.03 CD − 54.56 CE − 37.10 DE − 19.12 A2 − 4.11 B2 − 2.35 C2 + 11.03 D2 − 38.12 E2.
The Box-Cox plot shows the optimal transformation to normalize a set of data that is not normally distributed by specifying an appropriate Lambda (λ) for the model; hence it was evaluated to determine if any transformation was required to improve the normality of residuals. In this study, the Box-Cox graph for power transforms presented a Lambda value of 1 for the response variable, hence, demonstrating that the response does not require any transformation for B. bassiana Bgl production (Supplementary data). Thus, three-dimensional response surface plots (Fig. 4) were generated based on the CCD quadratic regression to demonstrate the pair-wise interactive effects of two variables and their effect on B. bassiana Bgl production. The z-axis in the 3D response surface plots represents the activity of Bgl as it relates to two specific variables while holding all other variables constant (Fig. 4). Interactions between AB, BE, CD, CE, and DE were observed to be significant with p value < 0.05, while the interactions between AC, AD, AE, BC, and BD were deemed to be insignificant due to p value > 0.05 Table 5. B. bassiana Bgl production was observed to increase with increasing concentration of Bambara and decreasing concentration of KCl (Fig. 4a). The interaction between NaCl and KCl as illustrated in Fig. 4b showed that Bgl production was favored at lower concentrations of both NaCl and KCl, while Bgl production decreased as both the concentrations were steadily increasing corroborating their negative effects on the Bgl production. Interaction between incubation time and all other tested variables indicated that as incubation time increased, Bgl production also increased, reaching a maximum level at 223 h; however, a gradual decline in enzyme production levels was observed beyond the optimum incubation time (Fig. 4c, e, and f). The decline in Bgl production after 223 h could be attributed to nutrient depletion which affected B. bassiana metabolism or the accumulation of proteases and other byproducts resulting in Bgl degradation as well as cell autolysis [43]. These results are corroborated by previous studies where enzyme production was also noted to have steeply declined beyond the optimum incubation time [19,20,21]. Interactions between incubation time and NaCl (CE) were more statistically significant amongst all other tested interactions in this study with the observed p value of 0.0003 (<0.05) (Fig. 4e). In addition, from the contour plots graphs, agitation speed between 140 and 150 rpm and NaCl concentration between 150 and 200 (mg/L) resulted in higher Bgl production (Fig. 4d). According to literature and the results obtained in this study, it can be inferred that further increase in Bgl production in different microorganisms is possible by optimization of medium components and physical components via statistical methods [38, 41].
3D-response surface plots for the β-glucosidase activity: a effect of KCl versus Bambara concentration, b effect of KCl versus NaCl concentration, c effect of incubation time versus KCl concentration, d effect of agitation speed versus NaCl concentration, e effect of incubation time versus NaCl concentration, and f effect of incubation time versus agitation speed
To validate the derived factorial model, the optimal values of the production factors predicted by CCD were concentrations of Bambara (57.03 g/L), KCl (302.81 mg/L), and NaCl (154.79 mg/L), as well as agitation speed of 150 rpm and incubation time of 223 h. The Bgl activity obtained with these predicted conditions was 711.0 U/mL, which was in close agreement with predicted values of 710.4 U/mL. This significant correlation between the predicted and obtained values demonstrates the adequate accuracy of RSM in the optimization of the fermentation parameters during B. bassiana Bgl production as well as validates the existence of optimum points. Through this statistical optimization approach, a 5.39-fold increase was recorded in Bgl production relative to the unoptimized level of 132.71 U/mL. These results are remarkable as the optimized Bgl yield recorded in this study is the highest level ever recorded from any entomopathogenic or endophytic fungus, thus, unlocking the potential of B. bassiana SAN01 as a viable source of Bgl.
3.4 Analysis of cellobiose hydrolysis by B. bassiana SAN01 β-glucosidase
The analysis of B. bassiana SAN01 Bgl hydrolysis products by thin-layer chromatography (TLC) demonstrated the capability of the enzyme to transform cellobiose into glucose. Glucose intensity was observed to increase with reaction time, while cellobiose intensity decreased with reaction time (Fig. 5a). The glucose released by the Bgl over time was further quantified and confirmed using HPLC. In this study, the absorption peak of glucose was observed at a retention time of 10.5 ± 0.4, which corresponded to the same retention time observed for the standard sugar used. Expectedly, the amount of glucose released from cellobiose increased with the incubation time and close to 100% of glucose was obtained after 24 h of incubation (Fig. 5b). The complete hydrolysis was observed after 24 h in this study, however, in previous studies by Verma et al. [44] and Pei et al. [33], (A) niger and Thermoanaerobacterium thermosaccharolyticum Bgls yielded 77% and 100% glucose within 12 h and 6 h, respectively. Hence, in order to achieve increased efficiency and enhance its industrial applications, it is imperative to optimize other factors involved in the hydrolytic process of the (B) bassiana Bgl - such as the pH, enzyme loading, substrate loading and incubation temperature.
a Thin-layer chromatography analysis of cellobiose hydrolysis by B. bassiana SAN01 β-glucosidase, M1: glucose standard, M2: cellobiose standard, lanes 3, 4, 5, 6, 7, and 8 show cellobiose hydrolysis after 0, 1, 6, 12, 18, and 24 h. b Cellobiose hydrolysis (blue line) and glucose production (red line) by B. bassiana SAN01 β-glucosidase as determined by HPLC
4 Conclusion
This study successfully optimized the production of Bgl by the novel endophytic fungus B. bassiana SAN01 via statistical modeling under submerged fermentation conditions. Initially, Bambara haulm was recorded as the biomass supporting the highest Bgl production; hence, it served as the major carbon source in the PDB and CCD experiments. At the end of the optimization process, an acceptable level of agreement was found between the experimental results and the statistical model. Bgl production improved by 5.39-fold compared to the unoptimized level, and according to our knowledge, the Bgl activity obtained in this study is the highest level ever recorded from any entomopathogenic or endophytic microbe. Thus, these results demonstrate the potential of statistical methods in optimizing the production medium for Bgl production by B. bassiana SAN01 while utilizing lignocellulosic biomass. The partially purified Bgl was successfully able to release glucose from cellobiose, thus indicating that Bgl can potentially be used in the hydrolysis of cellulose for biofuel production. However, it is considered important that further studies are required to elucidate the mechanism behind the significant production of Bgl with Bambara haulm as the carbon source, both at the micro level and molecular level. Furthermore, it is imperative to upscale the production of B. bassiana SAN01 Bgl to the bioreactor scale, as well as improve its saccharification capabilities via optimization and protein engineering, all with the aim of enhancing its real-time industrial applications.
Data availability
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bhati N, Shreya, Sharma AK (2021) Cost-effective cellulase production, improvement strategies, and future challenges. J Food Process Eng 44(2):e13623. https://doi.org/10.1111/jfpe.13623
Saroj P, Narasimhulu K (2022) Biochemical characterization of thermostable carboxymethyl cellulase and β-glucosidase from Aspergillus fumigatus JCM 10253. Appl Biochem Biotechnol 194(6):2503–2527. https://doi.org/10.1007/s12010-022-03839-2
Lv Y, Liu X, Zhou S, Yu Q, Xu Y (2022) Microbial saccharification–biorefinery platform for lignocellulose. Ind Crops Prod 189:115761. https://doi.org/10.1016/j.indcrop.2022.115761
Kannan P, Achudhan AB, Gupta A, Saleena LM (2023) A review of applications of β-glucosidase in food, brewery, pharmaceutical and cosmetic industries. Carbohydr Res 108855. https://doi.org/10.1016/j.carres.2023.108855
360iResearch (2021) Agricultural Enzymes market by type (beta-glucosidase, cellulases, dehydrogenases), product (growth enhancing products, soil fertility products), crop type - cumulative impact of COVID-19, Russia Ukraine Conflict, and high inflation - global Forecast 2022–2027. https://www.360iresearch.com/library/intelligence/agricultural-enzymes. Accessed on 22-09-2023
El-Ghonemy DH (2021) Optimization of extracellular ethanol-tolerant β-glucosidase production from a newly isolated Aspergillus sp. DHE7 via solid state fermentation using jojoba meal as substrate: purification and biochemical characterization for biofuel preparation. J Genet Eng Biotechnol 19(1):1–18. https://doi.org/10.1186/s43141-021-00144-z
Bertonha LC, Neto ML, Garcia JAA, Vieira TF, Castoldi R, Bracht A et al (2018) Screening of Fusarium sp. for xylan and cellulose hydrolyzing enzymes and perspectives for the saccharification of delignified sugarcane bagasse. Biocatal Agric Biotechnol 16:385–389. https://doi.org/10.1016/j.bcab.2018.09.010
Sun N, Liu X, Zhang B, Wang X, Na W, Tan Z et al (2022) Characterization of a novel recombinant halophilic β-glucosidase of Trichoderma harzianum derived from Hainan mangrove. BMC Microbiol 22(1):1–11. https://doi.org/10.1186/s12866-022-02596-w
Mule TA, Sawant SS, Odaneth AA (2024) Maize bran as a potential substrate for production of β-glucosidase. Biomass Conver Biorefin 14:4029–4039. https://doi.org/10.1007/s13399-022-02747-z
Senba H, Saito D, Kimura Y, Tanaka S, Doi M, Takenaka S (2023) Heterologous expression and characterization of salt-tolerant β-glucosidase from xerophilic aspergillus chevalieri for hydrolysis of marine biomass. Archives Microbiol 205:310. https://doi.org/10.1007/s00203-023-03648-z
Sun J, Wang W, Hao J (2024) A novel GH1 β-glucosidase from an Arctic bacterium: characterization and secretory expression in Bacillus subtilis. Process Biochem 140:108–116. https://doi.org/10.1016/j.procbio.2024.02.016
Frassatto PAC, Casciatori FP, Thoméo JC, Gomes E, Boscolo M, Da Silva R (2021) β-Glucosidase production by Trichoderma reesei and thermoascus aurantiacus by solid state cultivation and application of enzymatic cocktail for saccharification of sugarcane bagasse. Biomass Conver Biorefin 11:503–513. https://doi.org/10.1007/s13399-020-00608-1
Aytaş ZG, Tunçer M, Kul ÇS, Cilmeli S, Aydın N, Doruk T, Adıgüzel AO (2023) Partial characterization of β-glucosidase, β-xylosidase, and α-l-arabinofuranosidase from Jiangella alba DSM 45237 and their potential in lignocellulose-based biorefining. Sustain Chem Pharm 31:100900. https://doi.org/10.1016/j.scp.2022.100900
Golgeri M, Mulla DB, Bagewadi SI, Tyagi ZK, Hu S, Sharma A, Bilal S, Bharagava M, Ferreira RN, Gurumurthy LFR, D. M., Nadda AK (2024) A systematic review on potential microbial carbohydrases: current and future perspectives. Crit Rev Food Sci Nutr 64:438–455. https://doi.org/10.1080/10408398.2022.2106545
Amobonye A, Bhagwat P, Pandey A, Singh S, Pillai S (2020) Biotechnological potential of Beauveria bassiana as a source of novel biocatalysts and metabolites. Crit Rev Biotechnol 40(7):1019–1034. https://doi.org/10.1080/07388551.2020.1805403
Zimmermann G (2007) Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria Brongniartii. Biocontrol Sci Technol 17(6):553–596. https://doi.org/10.1080/09583150701309006
Amobonye A, Bhagwat P, Mthethwa N, Kwenda S, Ismail A, Kumari S et al (2023) Transcriptomic profiling of Beauveria Bassiana SAN01, an endophytic fungal entomopathogen, for the production of lignocellulosic enzymes. Biocatal Agric Biotechnol 102918. https://doi.org/10.1016/j.bcab.2023.102918
Dhaver P, Pletschke B, Sithole B, Govinden R (2022) Optimization, purification, and characterization of xylanase production by a newly isolated Trichoderma harzianum strain by a two-step statistical experimental design strategy. Sci Rep 12(1):17791. https://doi.org/10.1038/s41598-022-22723-x
Nisar K, Abdullah R, Kaleem A, Iqtedar M, Saleem F, Iftikhar T, Aslam F (2020) Application of Plackett–Burman and Box–Behnken design for the optimization of β-glucosidase production by thermophilic Thermomyces dupontii. Rev Chim 70(9):32–38. https://doi.org/10.37358/Rev.Chim.1949
Breig SJM, Luti KJK (2021) Response surface methodology: a review on its applications and challenges in microbial cultures. Mater. Today: Proc 42:2277-84. https://doi.org/10.1016/j.matpr.2020.12.316
Singh N, Sithole B, Kumar A, Govinden R (2023) A glucose tolerant β-glucosidase from a newly isolated Neofusicoccum parvum strain F7: production, purification, and characterization. Sci Rep 13(1):5134. https://doi.org/10.1038/s41598-023-32353-6
Baskaran R, Krishnan C (2020) Enhanced production of cellulase from a novel strain Trichoderma gamsii M501 through response surface methodology and its application in biomass saccharification. Process Biochem 99:48–60. https://doi.org/10.1016/j.procbio.2020.08.006
Amobonye A, Bhagwat P, Singh S, Pillai S (2021) Enhanced xylanase and endoglucanase production from Beauveria bassiana SAN01, an entomopathogenic fungal endophyte. Fungal Biol 125(1):39–48. https://doi.org/10.1016/j.funbio.2020.10.003
da Costa SG, Pereira OL, Teixeira-Ferreira A, Valente RH, de Rezende ST, Guimarães VM, Genta FA (2018) Penicillium citrinum UFV1 β-glucosidases: purification, characterization, and application for biomass saccharification. Biotechnol Biofuels 11(1):1–19. https://doi.org/10.1186/s13068-018-1226-5
Hartree E (1972) Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48:422–427. https://doi.org/10.1016/0003-2697(72)90094-2
Sorour AA, Olama ZA, El-Naggar MY, Ali SM (2023) Bioprocess development for extraction and purification of cellulases from Aspergillus niger 3ASZ using statistical experimental design techniques. Int J Biol Macromol 242:124759. https://doi.org/10.1016/j.ijbiomac.2023.124759
Sun N, Liu X, Wang X, Shi H, Zhang H, Li L, Na W, Guan Q (2021) Optimization of fermentation conditions for the production of acidophilic β-glucosidase by Trichoderma reesei S12 from mangrove soil. Biotechnol Biotechnol Equip 35(1):1838–1849. https://doi.org/10.1080/13102818.2021.1984989
Bhaturiwala R, Bagban M, Mansuri A, Modi H (2022) Successive approach of medium optimization using one-factor-at-a-time and response surface methodology for improved β-mannanase production from Streptomyces sp. Bioresour Technol Rep 18:101087. https://doi.org/10.1016/j.biteb.2022.101087
Boro M, Verma AK (2023) Optimization of cellulase production by Cohnella xylanilytica RU-14 using statistical methods. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-023-04447-4
Gautério GV, da Silva LGG, Hübner T, Ribeiro TR, Kalil SJ (2021) Xylooligosaccharides production by crude and partially purified xylanase from Aureobasidium pullulans: biochemical and thermodynamic properties of the enzymes and their application in xylan hydrolysis. Process Biochem 104:161–170. https://doi.org/10.1016/j.procbio.2021.03.009
Yepes C, Estévez J, Arroyo M, Ladero M (2022) Immobilization of an industrial beta-glucosidase from Aspergillus fumigatus and its use for cellobiose. Processes 10(6):1225. https://doi.org/10.3390/pr10061225
Kim H, Lee SJ, Shin K-S (2018) Characterization of new oligosaccharide converted from cellobiose by novel strain of Bacillus subtilis. Food Sci Biotechnol 27:37–45. https://doi.org/10.1007/s10068-017-0211-2
Pei J, Pang Q, Zhao L, Fan S, Shi H (2012) Thermoanaerobacterium thermosaccharolyticum β-glucosidase: a glucose-tolerant enzyme with high specific activity for cellobiose. Biotechnol Biofuels 5(1):1–10. https://doi.org/10.1186/1754-6834-5-31
Majola NG, Gerrano AS, Shimelis H (2021) Bambara groundnut (Vigna subterranea [L.] Verdc.) production, utilisation and genetic improvement in Sub-Saharan Africa. Agronomy 11(7):1345. https://doi.org/10.3390/agronomy11071345
Okuofu SI, Gerrano AS, Singh S, Pillai S (2022) Deep eutectic solvent pretreatment of Bambara groundnut haulm for enhanced saccharification and bioethanol production. Biomass Convers Biorefin 12(8):3525–3533. https://doi.org/10.1007/s13399-020-01053-w
Abdeshahian P, Kadier A, Rai PK, da Silva SS (2020) Lignocellulose as a renewable carbon source for microbial synthesis of different enzymes. Lignocellulosic Biorefin Technol 185–202. https://doi.org/10.1002/9781119568858.ch9
Kumar BA, Amit K, Alok K, Dharm D (2018) Wheat bran fermentation for the production of cellulase and xylanase by Aspergillus niger NFCCI 4113. Res J Biotechnol 13:5. https://doi.org/10.1016/j.jenvman.2022.114431
Huang C, Feng Y, Patel G, Xu X-q, Qian J, Liu Q (2021) Production, immobilization and characterization of beta-glucosidase for application in cellulose degradation from a novel Aspergillus versicolor. Int J Biol Macromol 177:437–446. https://doi.org/10.1016/j.ijbiomac.2021.02.154
Infanzón-Rodríguez MI, del Moral S, Castro-Martínez C, Cano-Sarmiento C, Gómez-Rodríguez J, Aguilar-Uscanga MG (2023) Multi-response optimization using the desirability function of exoglucanases, endoglucanases and β-glucosidases production by Aspergillus niger ITV-02 from delignified sugarcane bagasse. Sugar Tech 25(1):86–98. https://doi.org/10.1007/s12355-022-01191-7
Borgi I, Dupuy J-W, Blibech I, Lapaillerie D, Lomenech A-M, Rebai A et al (2016) Hyper-proteolytic mutant of Beauveria bassiana, a new biological control agent against the tomato borer. Agron Sustain Dev 36:1–9. https://doi.org/10.1007/s13593-016-0394-6
Singh N, Sithole BB, Govinden R (2023) Optimisation of β-glucosidase production in a crude aspergillus japonicus VIT-SB1 cellulase cocktail using one variable at a time and statistical methods and its application in cellulose hydrolysis. Int J Mol Sci 24(12):9928. https://doi.org/10.3390/ijms24129928
Amin M, Bhatti HN, Sadaf S, Bilal M (2021) Optimization of lipase production by response surface methodology and its application for efficient biodegradation of polyester vylon-200. Catal Lett 1–14. https://doi.org/10.1007/s10562-021-03603-x
Akula S, Golla N (2020) Significance of process parameters on fungal cellulase production. In: Srivastava N, Srivastava M, Mishra P, Gupta V (eds) Biofuel Production Technologies: critical analysis for sustainability. Clean Energy Production Technologies. Springer, Singapore, pp 299–324. https://doi.org/10.1007/978-981-13-8637-4_11
Verma ML, Chaudhary R, Tsuzuki T, Barrow CJ, Puri M (2013) Immobilization of β-glucosidase on a magnetic nanoparticle improves thermostability: application in cellobiose hydrolysis. Bioresour Technol 135:2–6. https://doi.org/10.1016/j.biortech.2013.01.047
Funding
Open access funding provided by Durban University of Technology. This work was supported by the National Research Foundation of South Africa under grant numbers (UID 138097, UID 146320, and UID 150503). The authors would like to thank the Durban University of Technology for funding the postdoctoral fellowship of the second author—Dr. Ayodeji Amobonye.
Author information
Authors and Affiliations
Contributions
Buka Magwaza: investigation, formal analysis, and writing—original draft; Ayodeji Amobonye: conceptualization, supervision, formal analysis, and review and editing; Prashant Bhagwat: investigation, formal analysis, and review and editing; Santhosh Pillai: conceptualization, resources, supervision, review and editing, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Magwaza, B., Amobonye, A., Bhagwat, P. et al. Hyper β-glucosidase producer Beauveria bassiana SAN01—optimization of fermentation conditions and evaluation of saccharification potential. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05866-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05866-x