Abstract
In the pursuit of cost-effective and superior enzymes crucial for the efficient hydrolysis of diverse lignocellulosic biomasses, filamentous fungi have emerged as key candidates for bioprospecting endeavors. In our exploration for potent lignocellulosic biomass degraders, we have identified a strain of Penicillium fuscoglaucum JAM-1, showcasing multipurpose hydrolase capabilities in its secretome. During fermentation, P. fuscoglaucum JAM-1 effectively utilized rapeseed cake (RSC), resulting in improved enzymatic activities, including xylanase (612 U/gds), β-glucosidase (264 U/gds), endoglucanase (102 U/gds), FPase (21.3 U/gds), and exo-polygalacturonase (49.17 U/gds), as compared to pine sawdust (PSD). Secretome profiling revealed a protein abundance totaling 435 and 120 proteins during RSC and PSD utilization, respectively. The major component of carbohydrate-active enzymes (CAZymes) consists of cellulose-degrading proteins, including endoglucanases (GH5, GH7), β-glucosidases (GH1, GH3, GH17), and cellobiohydrolases (GH6, GH7). Correspondingly, hemicellulose-degrading enzymes were present, encompassing endo-1,4-xylanase (GH10), α/β-galactosidase (GH27, GH35, GH36), α/β-mannosidases (GH38, GH2, GH47, GH5), and α-l-arabinofuranosidase (GH43, GH62, GH51, GH54) and carbohydrate-active auxiliary activities enzymes, such as AA9 (formerly known as GH61) lytic polysaccharide monooxygenase (LPMO). Upon application to fruit waste, the crude enzyme demonstrated higher saccharification potential compared to commercial cellulase (Cellic CTec2). Specifically, the crude enzyme yielded 565 mg/g of reducing sugar within 72 h, outperforming Cellic CTec2, which yielded 352 mg/g under identical conditions. A comprehensive comparative analysis of enzyme workings, activities, and secretome profiling underscores P. fuscoglaucum JAM-1 as a potent cellulase producer, showcasing its potential to boost lignocellulose biodegradation. These findings highlight the practical applications of the P. fuscoglaucum JAM-1 in various industrial processes, suggesting its role as a valuable candidate for further exploration and exploitation in biotechnological applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
By 2050, the global population is projected to expand by 10 billion, leading to heightened demand for essential resources such as food and energy [1, 2]. This surge in demand is expected to result in increased consumption of natural resources and consequences in the rise of waste generation, resulting in landfilling or uncontrolled dumping, causing environmental hazards and financial damage [3, 4]. Consequently, there is an urge for sustainable waste management on the awareness of a circular economy aimed at minimizing waste generation. In line with the above trends, there has been growing acknowledgment of the environmental repercussions of leftovers from lignocellulosic biomass, including agri-food processing and agroforestry. Agri-food processing debris includes byproducts of starch extraction, grains, legumes, fruits, vegetables, sugar production, and starch processing [5]. Correspondingly, agroforestry leftovers are lignocellulosic materials obtained from a variety of sources, including forests (wood chips and bark), the timber industry (sawdust), and agricultural crop wastes such as straw, husk (hull), bran, stalk, and bagasse [6]. Given these aspects, biowastes resulting from agroforestry and the agri-food processing industry have emerged as renewable and cost-effective carbon and nitrogen sources for producing value-added products such as enzymes, organic acids, food additives, and ethanol. However, because of the intrinsic difficulties due to the confined structure of cellulose, hemicellulose, and lignin in the lignocellulosic biomass, enzymatic hydrolysis of lignocellulose into marketable products became a complex process [7, 8]. So developing a practical approach (pretreatment) for facilitating monosaccharide production is essential. Among the array of available pretreatment methods, biological pretreatment is widely used. It employs microorganisms such as bacteria or fungi capable of secreting enzymes to break down cellulose, hemicellulose, and lignin [9, 10]. Bioconversion processes using bacteria, fungi, or their metabolites can reduce the costs of chemical transformation, increase specificity, and reduce toxicity and environmental pollution.
Nevertheless, fungi are the most commonly used microorganism preferred over bacteria in converting waste into valuable products due to their capability to secrete a diverse array of carbohydrate-degrading enzymes, multipurpose substrate utilization, and permeation ability [11]. Employing bacteria for enzyme production via submerged fermentation results in a low titer of the end product and contributes to the elevated cost of enzyme production due to additional purification and downstream processes [12, 13]. Consuming fungus-derived enzymes by solid-state fermentation (SSF) as a substitute production route for various industrial enzymes is an attractive approach to industries due to their wide range of applications for product generation with capital costs, high yield, and low waste. In recent decades, dynamic studies have been conducted to achieve efficient and cost-effective enzyme production methods for degrading lignocellulosic biomass. The market value rises daily, reaching between $0.23 and $0.78 per gallon of ethanol and higher, depending on factors such as enzyme source, pretreatment process, and production approach [14, 15]. Reduction in enzyme costs has only been possible through discovering newly efficient and stable enzymes.
As a result, ongoing researchers are trying to identify multipurpose enzymes derived from a single microbe sufficient for all types of lignocellulosic biomass hydrolysis without the need for purification. This approach could make it more economically competitive, improving existing enzyme capabilities and designing appropriate enzyme blends, which are critical steps toward addressing one of the barriers (enzyme cost) impeding the commercialization of lignocellulose biorefineries [16, 17].
To address the bottleneck of enzyme versatility in hydrolyzing diverse biomass due to structural recalcitrance and inhibitor presence, this study was designed to investigate the utilization of two substrates by indigenously isolated filamentous fungi (Penicillium fuscoglaucum JAM-1) from fruit waste. We aim to obtain a single versatile enzyme producer capable of efficiently hydrolyzing various biomass substrates. We intended to gain significant knowledge regarding the CAZyme repertoire and its abundance within the secretome utilizing different substrates. Carrying out secretome analysis allows for identifying the array of enzymes that a fungus can produce in the presence of various substrates, facilitating advancements in bioconversion research. Moreover, we aimed to compare the efficacy of crude enzymes from P. fuscoglacum JAM-1 in saccharification of fruit waste (orange peel) with commercially available Cellic CTec2 enzyme. This comparison will assess the potential of fungus-derived enzymes in generating fermentable sugars to meet commercial demands and validate a circular economy approach for various high-value products.
2 Materials and methods
2.1 Growth conditions and molecular identification of a filamentous fungus
The filamentous fungus used in this study was isolated from fruit waste, mainly mixed citrus fruit obtained from a local market in Poland. The samples were grown on Reese’s mineral medium, specification given in Joshi et al. [18], with antibiotics (ampicillin) used to restrict bacterial growth supplemented with 1% carboxy methyl cellulose (CMC), incubated at 25 °C temperatures for 7 days. The isolated cultures were purified through repeated sub-culturing and stored at 4 °C. The isolated strains from fruit waste underwent molecular characterization using 18 S rDNA-based sequencing using universal primers ITS1 (5′ CCGTAGGTGAACCTGCGG 3′) and ITS4 (5′ TCCTCCGCTTATTGATATG 3′) sequenced at Eurofins Polska (https://www.eurofins.pl/). The sequenced product was then BLAST searched at the National Center for Biotechnology Information (NCBI) database, and evolutionary distances were analyzed using the molecular evolutionary genetics analysis (MEGA 11) tool, creating a phylogenetic tree using the neighbor-joining method [19].
2.2 Microbial inoculant, substrates, and chemicals
The identified fungus P. fuscoglaucum JAM-1 was freshly cultured for plates for 7 days at 25 °C for spore collection. Later, fungal spores and mycelia were gently scraped in 1% tween-20 to make a sterile spore suspension, which was then stored at 4 °C for media inoculation. Dried rapeseed cake (RSC) after oil extraction and pine sawdust (PSD) were locally arranged from the countryside of Poland, finely chopped, and sieved with 1 mm mesh size. Both substrates were dried and stored at room temperature (~ 22 °C) for future studies. Utilizing dried substrates ensures precise weighing protocols and strengthens experimental integrity by minimizing the risk of contamination. By following standard National Renewable Energy Laboratory (NREL) procedures, the chemical composition of untreated PSD and RSC (% w/w) was ascertained to be 44.8 ± 0.2 and 23.2 ± 0.3 of cellulose, 27.9 ± 0.4 and 42.6 ± 0.5 of hemicelluloses, and 23.9 ± 0.4 and 14. ± 0.25 of lignin [20]. Other substrates including carboxymethyl (CM) cellulose, citrus peel pectin (P9135), Whatman® qualitative filter paper, Grade 1 (WHA1001090), and p-nitrophenyl-β-d-glucopyranoside (N7006) were obtained from Merck Life Science Sp.z.o.o., an affiliate of Merck KGaA, Darmstadt, Germany (Poznań, Poland). The commercial cellulase enzyme utilized in this study, Cellic CTec2 (SAE0020), was also obtained from Sigma-Aldrich (Merck). Beechwood xylan was acquired from Megazyme (Neogen Europe Ltd., UK), which is a 3,5-dinitrosalicylic acid (DNS) (609,994) reagent acquired from Alfa Aesar. Standards such as d-(+)-glucose, d-(+)-xylose, and d-(+)-galacturonic acid monohydrate were sourced from Sigma-Aldrich (Merck). All other chemicals employed were of analytical grade.
2.3 Solid-state fermentation (SSF) of lignocellulosic substrates for enzyme production
Five grams of a substrate (RSC and PSD) was moistened with Reese’s mineral medium in the substrate-to-moisture ratio 1:3, followed by autoclaving at 121 °C. After sterilization, 108 spores per gram were inoculated and incubated (Memmert Incubator INB 400, Memmert GmbH, Germany) at 25 °C under static conditions for 7 days. Following fermentation, enzyme extraction was performed using 50 ml of 0.05 M citrate buffer (pH 4.8), stirred at 150 rpm for 1 h. The extracellular enzyme was separated from the biomass by centrifugation (Centrifuge 5804 R, Eppendorf Poland Sp.z o.o) at 8000 rpm for 20 min, and the supernatant was collected for further analysis.
2.4 Determining enzymatic activities
Extracellularly produced enzymes were used to determine the different enzymatic activities. Cellulase (CMCase and FPase) activity was assessed using 2% (w/v) carboxymethyl cellulose (CMC) and 50 mg Whatman No. 1 filter paper dissolved in 0.05 M citrate buffer (pH 4.8), along with a 50 times diluted enzyme. The CMCase and FPase activity mixture was incubated at 50 °C for 30 min and 60 min, respectively. Likewise, xylanase activity was estimated by dissolving 1% (w/v) beechwood xylan in 0.05 M citrate buffer (pH 4.8). The reaction condition for xylanase activity involved a 30-min incubation period at 50 °C. Following the incubation for all three enzymes, i.e., FPase, CMCase, and xylanase, the total amount of reducing sugar was determined using the DNS method at 540 nm [21]. Under the given assay conditions, one unit of FPase, endoglucanase, and xylanase activity was defined as the amount of enzyme that released 1 µmol of reducing sugar (glucose/xylose) per minute. The β-glucosidase activity was also measured using 5 mM p-nitrophenyl-β-d-glucopyranoside (pNPG) dissolved in 0.05 M citrate buffer (pH 4.8) and terminated by adding glycine buffer (pH 10.8). The incubation condition is similar to the activity mentioned above (endoglucanase). The amount of product was measured at 405 nm [22]. One unit of β-glucosidase activity was defined as the quantity of enzyme capable of liberating 1 µmol of p-nitrophenol (pNP) per minute using extinction coefficient as 15,200 M−1·cm−1. Moreover, exo-polygalacturonase activity was determined by adding a diluted enzyme solution (100 µl) to a reaction mixture containing pectin solution (0.5%) in 0.05 M citrate buffer (pH 4.8), followed by a 30 min incubation at 40 °C. The reaction was stopped by adding a DNS reagent and heating it in a boiling water bath for 10 min. The reducing sugars liberated in the solution were measured at 540 nm using d-galacturonic acid monohydrate as the standard [23].
2.5 Analytical method for determining different volatile compounds by GC-MS
The organic compounds were separated using an Agilent 7890 A series gas chromatograph, interfaced with a mass selective detector, and an Agilent 7683 series injector (Agilent Technologies, USA), injecting a 5 µl sample with a split ratio of 1:5 (sample to carrier gas) by 0.3% SD as achieved on an HP-5MS column (Agilent Technologies, USA), with helium serving as the carrier gas at a flow rate of 1 ml min−1. The GC oven was programmed with a temperature gradient, initiating at 100 °C (5 min) and gradually increasing to 280 °C (5 min) at a rate of 8 °C min−1. Mass spectrometry analysis was performed in the electron-impact mode at an ionizing potential of 70 eV, recording mass spectra from m/z 40 to 800.
To extract additional organic compounds, 100 ml of cell-free aqueous phase samples were mixed with 25 ml of chloroform in a separatory funnel for 3 min, repeated thrice. The remaining aqueous phase, post-chloroform extraction, underwent a similar extraction process with hexane. The resulting organic extracts were combined and dried with anhydrous Na2SO4, and the solvent was evaporated under a stream of N2. Subsequently, the samples were derivatized with 0.5 ml of N,O-bis(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane (Supelco, USA) for 30 min at 70 °C. A blank sample was prepared following the same procedure. The peaks that exhibited mass spectra matching or surpassing 50% similarity with reference spectra were recognized and selected for further analysis.
2.6 Enzymatic saccharification of orange peel
Oranges were procured locally from the Warsaw market, peeled at the laboratory, and further used for the enzymatic hydrolysis process; that is, 10% (w/v) of the orange peel biomass was diluted using a 0.05 M sodium citrate buffer (pH 4.8). Orange peel contains 6% lignin, 46% cellulose and hemicellulose, and 40% pectin [24]. The enzyme loading was optimized to 15 U, 30 U, and 45 U/gds, and the saccharification was carried out at 37 °C with continuous agitation at 150 rpm for 72 h. Similar enzyme loading was performed using a commercial enzyme (Cellic CTec2 enzyme). The amount of reducing sugars released into the supernatant was determined using the DNS method [21].
2.7 Secretome analysis of P. fuscoglaucum JAM-1 grown over the lignocellulosic substrates
The extracellular enzyme extracted from the P. fuscoglaucum JAM-1 during SSF of lignocellulosic substrates, i.e., RSC and PSD, was centrifuged and subjected to the acetone precipitation method using chilled acetone for 3–4 h at a cold temperature. After centrifugation, the pellet was air-dried and dissolved in 0.05 M citrate buffer (pH 4.8) for further use. The acquired (20 µg) protein was placed into 12% SDS-PAGE and separated by slicing into 3–4 gel sections. A solution of 75% acetonitrile and 25 mM ammonium bicarbonate buffer was used to wash and destain each cut gel. Dithiothreitol (10 mM) was used as a reducing agent, while iodoacetamide (55 mM) was used as an alkylating agent. The alkylated protein was subsequently digested overnight with trypsin at 37 °C. The tryptic digest was then extracted with acetonitrile and 1% formic acid before being analyzed using liquid chromatography (LC) measurement of the masses in a mass spectrometer (MS). The samples were qualitatively analyzed at the Laboratory of Mass Spectrometry, Institute of Biochemistry and Biophysics Polish Academy of Sciences (IBB PAS), Warsaw, Poland. For protein analysis, the Mascot 2.4.01 search engine (https://www.matrixscience.com/) was used by listing the raw sequence searched against the UniProt database for Penicillium species by arranging peptide tolerance to ∓ 19 ppm and fragment ion mass tolerance to ∓ 0.01 Da and permitted one missed cleavage. Carbamidomethylation and methionine oxidation were used as fixed and variable modifications, respectively. The isoelectric point (pI) and molecular weight (MW) were analyzed to identify each protein using the Compute pI/Mw tool in ExPASy (http://web.expasy.org/compute_pi). Other findings, such as carbohydrate and auxiliary-active enzyme families, were assessed using the CAZy database (http://www.cazy.org; [25]).
2.8 Statistical analysis
All experiments were performed in triplicates. The values in graphs and tables are the mean ± SD of three replicates determined in MS Excel. Custom scripts have been used to prepare graphs using R, the pheatmaps library has been used to construct heatmap, the ggplot library has been used to create scatter plots, and the VennDiagram library has been used to perform Venn analysis.
3 Results and discussions
3.1 Molecular characterization of isolated fungus
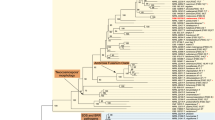
For isolated strains from food waste, 18 S rDNA-based sequencing was used for molecular characterization. The ITS region was subjected to amplification and sequencing procedures. Subsequent sequence data alignment and analysis were conducted to ascertain the closest matches within the National Center for Biotechnology Information (NCBI) database. BLAST analysis indicated significant similarity with Penicillium fuscoglaucum and was further submitted to the NCBI database under the accession number OM258158. The phylogenetic tree was constructed with the neighbor-joining method (Fig. 1) and included reference strains from the NCBI GenBank, highlighting the close evolutionary relationship among Penicillium species.
3.2 Hydrolytic activity of the extracellular enzyme produced during solid-state fermentation (SSF)
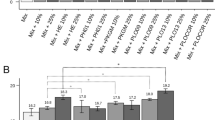
In SSF, out of both substrates, the presence of RSC during fermentation resulted in xylanase (612 U/gds), β-glucosidase (264 U/gds), endoglucanase (102 U/gds), FPase (21.3 U/gds), and exo-polygalacturonase activity (49.17 U/gds). While with PSD as a substrate, relatively low enzymatic activity compared to RSC, resulted in 525 U/gds for xylanase, 86 U/gds for β-glucosidase, 51.4 U/gds for endoglucanase, 9.14 U/gds for FPase, and 29.86 U/gds for exo-polygalacturonase activity, respectively (Fig. 2). The observed disparity concerning the enzymatic activity of both substrates can be ascribed to the substantial nutritional composition. Notably, RSC features a significant protein content ranging from 14-40%, 5–15% of crude fiber, and an approximate total carbon content of 50% (w/w) [26, 27]. These constituents position RSC as a viable substrate in SSF, serving as a pivotal carbon source for the growth of fungal strains. Conversely, due to its intricate and rigid structure, the presence of phenolic and resinous chemicals derived from lignin in PSD may impede enzymatic activity during SSF, rendering it less conducive to the growth of fungi [28].
Comparative analysis of the enzymatic activity of cellulases, xylanases, and pectinase in SSF using RSC and PSD by P. fuscoglaucum JAM-1. The enzyme activities were estimated after 7 days of incubation. The values represent the mean of three replicates, and the error bars represent standard deviations from the mean
In the study conducted by Sousa et al. [27], the use of rapeseed cake in conjunction with Aspergillus niger demonstrated the maximum cellulase activity at 109 U/gds and xylanase activity at 692 U/gds. Similarly, employing a microbial consortium yielded enhanced cellulase and xylanase activities, reaching 135 U/gds and 886 U/gds, respectively [29]. Since the titer of enzyme production varies from substrate to substrate, Table 1 summarizes the comparative activity of enzymes extracted from Penicillium sp. with different lignocellulosic substrates as carbon sources. Earlier studies by Su et al. [30] and Kumar et al. [31] confirmed that utilizing wheat bran as a carbon source resulted in higher cellulase and xylanase activity production. This enhanced enzyme activity was attributed to exogenous nutrient supplementation. Utilizing wheat bran as a supplement results in its spoilage before human consumption, which drives up expenses and consequently generates competition within the food feed sector [32]. However, P. fuscoglaucum JAM-1 utilized RSC and PSD to produce crude enzymes without requiring extra supplementation, allowing for the cost-effective manufacturing of the products. Resulting in an evaluation of enzymatic activities, the residual supernatant was further selected for GC-MS analysis on the seventh day after harvest.
3.3 Examining lignocellulosic biomass for bio-based compounds during SSF
The SSF process has gained substantial interest in producing bio-based compounds from widespread and resistant lignocellulosic feedstocks. Significant categories were examined in the current study, comprising 6–10% alcohols, 46% organic acids/esters, 10–20% aliphatic compounds, 2–3% aromatic compounds, and the remaining percent for other compounds. These significant categories include various bio-compounds, including volatile fatty acids (VFAs), aromatic compounds, phenolic compounds, and aroma and flavor compounds based on substrate rapeseed and sawdust, as shown in Fig. 3a, b and Table S1 and S2. VFA contains acetic acid, butyric acid, propionic acid, and their iso-forms. VFA has many applications, including livestock feed, beauty products, chemical solvents, adhesives and related products, nutrition, bio-energy, and pharmaceuticals [36]. Until now, various aromatic chemicals, primarily benzoic acid and phenylpropanoid derivatives of cinnamic acid, have been synthesized from edible and non-edible biomass. These substances are the building blocks for aromatic bio-based polymers [37]. According to Chong et al. [38], several bio-lipids, such as palmitic acid, palmitoleic acid, and oleic acid, are generated after lignin degradation.
Sunburst diagram of various bio-compounds formed during the SSF of P. fuscoglaucum JAM-1 using two different substrates, a RSC and b PSD. The compounds are named according to their chemical structures. For clarity, both the full names and abbreviations of some compounds are provided, such as 4-Cl-3-NO2-N-Ph-BSA (4-chloro-3-nitro-N-phenylbenzenesulfonamide), N-Tert-Bu-2-BTSA (N-tert-butyl-2-benzothiazolesulfenamide), 7-Cl-4-NO2-BODA (7-chloro-4-nitro-2,1,3-benzoxadiazol-5-amine), 3-Me-5-Ph-2,4-IMD (3-methyl-5-phenyl-2,4-imidazolidinedione), 6-Br-2,3-DCN (6-bromo-2,3-dicyanonaphthalene), BDTMG (bisdethiobis(methylthio)gliotoxin), 5-Br-2-EOPA (5-bromo-2-ethoxypyridin-3-amine), 1 H-Pz-3-CA (1 H-pyrazole-3-carboxylic acid), TMS-HO-NH2 (O-(trimethylsilyl)hydroxylamine), 5-Br-2-EOPA (5-bromo-2-ethoxypyridin-3-amine), P2E-3,3-Me2-I2O (prop-2-enyl-3,3-Me2-indol-2-one), 1 H-Pz-3-COOH (1 H-pyrazole-3-carboxylic acid), 3-CPCP-1-one (3 cyclopropylcyclopentan-1-one), dodecyl 4-NP ether (dodecyl 4-nitrophenyl ether), 2-Am-5-Fl-3-NO2-BA (2-amino-5-fluoro-3-nitrobenzoic acid), 3,4,5-TMBA (3,4,5-trimethoxybenzoic acid), CDC acid (cyclododecanecarboxylic acid), EMTP (ethyl 2-(methylthio)propanoate), CDDCA (cyclododecanecarboxylic acid), BFC acid (benzofuran-4-carboxylic acid), 5-(CF3)Thz-2-amine (5-(trifluoromethyl)thiazol-2-amine), 5-AICAR (5-aminoimidazole-4-carboxamide), HICA (2-hydroxyisocaproic acid), MEMA (monoethyl methylmalonate), KIV (alpha-ketoisovaleric acid), CTDC acid (cyclotetradecanecarboxylic acid), PGMA (propylene glycol monoacetate), 3-MeOPAA (3-methoxyphenylacetic acid), 3-Me-Ind-1-yl-PrA (3-Me-indol-1-yl-propylamine), dodecyl 4-NPE (dodecyl 4-nitrophenyl ether), 2-Br-2-MP (2-bromo-2-methylpropanal), 2,4-CHD-1-one (2,4-cycloheptadien-1-one), P3CA (pyrazole-3-carboxylic acid), HICA (2-hydroxyisocaproic acid), TMSU (trimethylsilyl undecanoate)
Similarly, these bio-lipids in both RSC and PSD indicate that P. fuscoglaucum JAM-1 has the ability to break down lignin. Furthermore, among the producers of value-added compounds labeled “natural,” bioflavors (flavors and aromas) are attempting to increase product adoption by the consumer market. These compounds, including alcohols, ketones, aromatic compounds, aldehydes and esters, and pyrazines, are remarkable for their volatility and chemical diversity [39, 40]. The present investigation encompasses a range of chemical substances, such as vanillin, isobutyl acetate, propyl acetate, isoamyl acetate (3-methylbutyl acetate), and propionate. The flavor and aroma characteristics of these compounds are significantly esteemed; a similar observation is supported by Hadj Saadoun et al. [41] and Berger [42]. These developments would benefit industries such as pharmaceuticals, agriculture, and biofuels and contribute to sustainable development by reducing reliance on fossil fuels and minimizing environmental impact. Additionally, developing robust biocatalysts could open up new avenues for producing complex molecules that are currently challenging or expensive to synthesize through traditional chemical methods.
3.4 Saccharification of orange peel
The reducing sugars in orange peel were quantified using crude enzymes derived from P. fuscoglaucum JAM-1, which was grown using RSC and PSD as the only carbon substrates. Due to the low lignin level in orange peels, no alkaline pretreatment was involved. The saccharification procedure encompassed enzyme loadings ranging from 15 to 45 U/gds, highlighting superior saccharification efficiency at the 15 U enzyme concentration. The experimentation comprised three primary sets: employment of a commercial enzyme (Cellic CTec2), utilization of enzymes extracted from RS, and employment of enzymes extracted from PSD with saccharification transpired over 24, 48, and 72 h at 37 °C. Notably, the enzyme derived from RSC displayed the highest substrate saccharification, yielding a 1.6-fold increase in reducing sugar production compared to the commercial enzyme (Fig. 4). The reason for achieving higher reducing sugar (565 mg/g of orange peel) could be the presence of different CAZymes, including pectinase, which allows the improved saccharification of the orange peel than the Cellic CTec2 enzyme.
In contrast, the enzyme from PSD demonstrated nearly equivalent reducing sugar yield (352 mg/g of orange peel) relative to the commercial enzyme. Su et al. [30] reported that the application of Penicillium roqueforti led to the generation of reducing sugars amounting to 259.45 mg/g from sugarcane bagasse. A comparable outcome was observed using Penicillium waksmanii F10-2 on wheat straw, resulting in 350.03 mg/g [43].
3.5 Comparative extracellular proteomic analysis between RS and SD
Secretome analysis of substrates (RSC and PSD) was performed using LC-MS/MS to identify the diversity of different proteins. A total of 435 and 120 proteins were identified in the P. fuscoglaucum JAM-1 secretome analysis using RSC and PSD, respectively, with 52 of the total proteins being common in both secretome analyses, as shown by the Venn diagram (Fig. 5a, Table S3 and S4). All identified proteins in both substrates had molecular weights ranging from 5.783 to 156.86 kDa, and their pI ranged from acidic to basic (3.78 to 10.23), with the majority being acidic, as shown in Fig. 5b. CAZymes are the major protein group present in the filamentous fungus, recognized in the P. fuscoglaucum JAM-1 secretome, and similar observation was reported by Tavares et al. [44]. Of the total proteins, 153 and 38 were identified as CAZymes using RSC and PSD, among which 17 were common in both secretomes (Fig. 5c). Among these shared proteins, the two predominant presence was observed for endo-1,3-β-glucosidase (GH17), which exhibited higher abundance in the RSC secretome with an exponentially modified protein abundance index (emPAI) of 5.24 and 1.08, followed by β-galactosidase (GH35) and β-xylanase (GH10; Table 2). In RSC, 153 CAZymes were further subcategorized to contribute 35% of all identified proteins, including 27% glycosyl hydrolases (GH); 3% auxiliary activities (AA); 2% carbohydrate esterases (CE), including lipase; and 3% polysaccharide lyase (PL). Accessory proteins included 5% oxidoreductase, 7% peptidases, 1% carbohydrate binding, and 52% unknown proteins with other functions (Fig. 6a). Similar results can be seen in Fig. 6b with PSD, where 38 CAZymes out of 120 identified proteins comprise 28%, 2%, 2%, and 2% of GH, AA, GT, and PL, respectively. Other proteins account for 5%, 6%, and 55%, including oxidoreductase, peptidases, and unidentified proteins, respectively, with other functions.
Distribution of proteins secreted during RSC and PSD secretome analysis in SSF using P. fuscoglaucum JAM-1. a The relative distribution of proteins in both substrates; b categorization of proteins based on MW and pI, respectively; c Venn diagram showing the distribution of CAZymes secreted by both substrates
Functional classification of proteins identified in the secretome of P. fuscoglaucum JAM-1 percentage wise. a Indicating the presence of protein in RSC; b indicating the presence of protein in PSD; c heatmap of proteins present in the different substrate-related secretomes of P. fuscoglaucum JAM-1 showing the abundance of differentially associating proteins. CAZyme families marked with ** contain members acting on other polysaccharides as well
Furthermore, a visual representation of the distribution of different CAZyme families and the relative abundance of the significant proteins detected across the different secretomes of rapeseed and sawdust is shown in Fig. 6c. Utilizing rapeseed secreted forty-seven cellulases, forty-six hemicellulases, six ligninases, and twenty-five pectinases, while with sawdust used as a substrate, the corresponding enzymes were sixteen cellulases, twelve hemicellulases, one ligninase, and five pectinases. Furthermore, P. fuscoglaucum stood out in the production of endoglucanases (GH5, GH7), β-glucosidases (GH1, GH3, GH17), and cellobiohydrolases (GH6, GH7) when rapeseed was utilized as a substrate. Notably, hemicellulases were also found to be abundant in the secretome profiling of JAM-1 when RSC was used, encompassing diverse proteins such as endo-1,4-xylanase (GH10), α/β-galactosidase (GH27, GH35, GH36), α/β-mannosidases (GH38, GH2, GH47, GH5), and α-l-arabinofuranosidase (GH43, GH62, GH51, GH54), thereby underscoring the broad enzymatic repertoire employed during substrate utilization. AA9-Lytic polysaccharide monooxygenases (LPMOs), formerly known as GH61, were detected: three in rapeseed and one in. These enzymes play a crucial role in efficient cellulose hydrolysis. However, oxidoreductases that could be involved in lignin degradation were classified as auxiliary enzymes (AA1, AA3, and AA4) found in both substrate secretomes. As per the findings of Corrêa et al. [45], AA4 (vanillyl alcohol oxidase) may play a role in the transformation of lignin-derived compounds (primarily alcohols) into aldehydes, ketones, and lactones. Additionally, Huang et al. [46] noted that a broad range of enzymes are required to degrade pectin, as it is a complex structure associated with cellulose. In the present study, P. fuscoglaucum JAM-1 secreted pectin-degrading enzymes with higher abundance in RSC with the presence of pectate lyases (PL1, PL3), polygalacturonase (GH28, GH53) rhamnogalacturonan lyase (PL4) responsible for pectin degradation. The introduction of pectinase into the enzymatic extract of Trichoderma harzianum has previously been shown to enhance the saccharification of pretreated sugarcane bagasse [47]. Similarly, in the JAM-1 secretome also, various enzymes responsible for starch degradation, including α-amylases (GH13) and alpha-glucosidase (GH31, GH15), were secreted [48].
Moreover, the abundance of CAZyme families was substantially higher in RSC than in PSD as a substrate. This distinction became particularly evident when comparing RSC’s cellulolytic and pectinolytic activities during SSF, which were significantly higher than those of PSD as a substrate during SSF. Furthermore, certain enzymes, such as carbohydrate esterases (CE), including lipase, a carbohydrate-binding enzyme, are exclusively found in RSC. The substrate, like RSC, contains a residual amount of fats and fatty acids, which may be acted upon by the extracellular lipase produced during the fermentation process [49, 50]. The remaining unexplored realm of uncharacterized proteins in proteome assays presents a promising avenue for discovering novel and exceptional activities related to biomass degradation.
Overall, the study’s findings underscore the considerable improvements in saccharification achieved through the varied array of hydrolases and auxiliary enzymes in the JAM-1 secretome. This suggests that producing hydrolases sustainably and cost-effectively by valorizing substrates such as RSC and PSD holds promise for diverse industrial applications. However, it is essential to recognize the potential challenges and limitations that may arise in real-world applications, including substrate variability, enzyme stability, and process robustness, which could impact the consistency and efficiency of biomass conversion processes at a commercial scale. Nonetheless, utilizing low-cost agro-industrial residues as substrates, without the need for extraction prior to saccharification, enhances the sustainability of the process compared to alternative methods.
4 Conclusion
Our study highlights the importance of the potential of P. fuscoglaucum JAM-1 as an efficient cellulase producer for the degradation of lignocellulosic biomasses, specifically RSC and PSD. The remarkable hydrolase capabilities exhibited by JAM-1, as evidenced by elevated enzymatic activities during RSC fermentation, underscore its suitability for cost-effective enzyme production. The secretome profiling revealed a rich repertoire of CAZymes, focusing on cellulose-degrading proteins, including endoglucanases, β-glucosidases, and cellobiohydrolases. Notably, the higher protein abundance observed during RSC utilization further emphasizes the strain’s proficiency in utilizing this substrate. The improved saccharification potential demonstrated by the crude enzyme derived from RSC, exceptionally outperforming commercial cellulase when applied to orange peel, underscores the practical applications of P. fuscoglaucum JAM-1 in various industrial processes. The comprehensive comparative analysis of enzyme components, activities, and secretome profiling provides valuable insights into the strain’s versatility and effectiveness in biomass degradation.
References
Gupta J, Kumari M, Mishra A et al (2022) Agroforestry waste management- a review. Chemosphere 287:132321. https://doi.org/10.1016/j.chemosphere.2021.132321
Searchinger T, Waite R, Hanson C et al (2019) Creating a sustainable food future: a menu of solutions to feed nearly 10 billion people by 2050. Final report. https://agritrop.cirad.fr/593176/1/WRR_Food_Full_Report_0.pdf
Liguori R, Faraco V (2016) Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour Technol 215:13–20. https://doi.org/10.1016/j.biortech.2016.04.054
Allesch A, Brunner PH (2014) Assessment methods for solid waste management: a literature review. Waste Manag Res 32:461–473. https://doi.org/10.1177/0734242X14535653
Ajila CM, Brar SK, Verma M et al (2012) Bio-processing of agro-byproducts to animal feed. Crit Rev Biotechnol 32:382–400. https://doi.org/10.3109/07388551.2012.659172
Abdeshahian P, Ascencio JJ, Philippini RR et al (2021) Valorization of lignocellulosic biomass and agri-food processing wastes for production of glucan polymer. Waste Biomass Valoriz 12:2915–2931. https://doi.org/10.1007/s12649-020-01267-z
Singhvi MS, Gokhale DV (2019) Lignocellulosic biomass: hurdles and challenges in its valorization. Appl Microbiol Biotechnol 103:9305–9320. https://doi.org/10.1007/s00253-019-10212-7
Yan J, Oyedeji O, Leal JH et al (2020) Characterizing variability in lignocellulosic biomass: a review. ACS Sustainable Chem Eng 8:8059–8085. https://doi.org/10.1021/acssuschemeng.9b06263
Li X, Shi Y, Kong W et al (2022) Improving enzymatic hydrolysis of lignocellulosic biomass by bio-coordinated physicochemical pretreatment—a review. Energy Rep 8:696–709. https://doi.org/10.1016/j.egyr.2021.12.015
Kumar Saini J, Himanshu H et al (2022) Strategies to enhance enzymatic hydrolysis of lignocellulosic biomass for biorefinery applications: a review. Bioresour Technol 127517. https://doi.org/10.1016/j.biortech.2022.127517
Yang Y, Yang J, Liu J et al (2018) The composition of accessory enzymes of Penicillium Chrysogenum P33 revealed by secretome and synergistic effects with commercial cellulase on lignocellulose hydrolysis. Bioresour Technol 257:54–61. https://doi.org/10.1016/j.biortech.2018.02.028
Srivastava N, Srivastava M, Mishra PK et al (2018) Applications of fungal cellulases in biofuel production: advances and limitations. Renew Sustain Energy Rev 82:2379–2386. https://doi.org/10.1016/j.rser.2017.08.074
Yoon LW, Ang TN, Ngoh GC, Chua ASM (2014) Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass Bioenergy 67:319–338. https://doi.org/10.1016/j.biombioe.2014.05.013
Sakhuja D, Ghai H, Rathour RK et al (2021) Cost-effective production of biocatalysts using inexpensive plant biomass: a review. 3 Biotech 11:280. https://doi.org/10.1007/s13205-021-02847-z
Adsul M, Sandhu SK, Singhania RR et al (2020) Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enzyme Microb Technol 133:109442. https://doi.org/10.1016/j.enzmictec.2019.109442
Liang C, Wang Q, Wang W et al (2023) Enhancement of an efficient enzyme cocktail from Penicillium consortium on biodegradation of pretreated poplar. Chem Eng J 452:139352. https://doi.org/10.1016/j.cej.2022.139352
Jørgensen H, Pinelo M (2017) Enzyme recycling in lignocellulosic biorefineries. Biofuel Bioprod Biorefin 11:150–167. https://doi.org/10.1002/bbb.1724
Joshi N, Grewal J, Drewniak L, Pranaw K (2024) Bioprospecting CAZymes repertoire of aspergillus fumigatus for eco-friendly value-added transformations of agro-forest biomass. Biotechnol Biofuels Bioprod 17:3. https://doi.org/10.1186/s13068-023-02453-6
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. https://doi.org/10.1093/molbev/msab120
Sluiter A, Hames B, Ruiz R et al (2008) Determination of structural carbohydrates and lignin in biomass. Lab Anal Procedure 1617(1):1–16
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. In: Methods in enzymology. Academic Press 160:87–112. https://doi.org/10.1016/0076-6879(88)60109-1
Amin F, Asad SA, Nazli Z-I-H et al (2023) Immobilization, biochemical, thermodynamic, and fruit juice clarification properties of lignocellulosic biomass–derived exo-polygalacturonase from Penicillium paxilli. Biomass Convers Biorefinery 13:13181–13196. https://doi.org/10.1007/s13399-022-02559-1
Utekar PG, Kininge MM, Gogate PR (2021) Intensification of delignification and enzymatic hydrolysis of orange peel waste using ultrasound for enhanced fermentable sugar production. Chem Eng Process - Process Intensif 168:108556. https://doi.org/10.1016/j.cep.2021.108556
Drula E, Garron M-L, Dogan S et al (2022) The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res 50:D571–D577. https://doi.org/10.1093/nar/gkab1045
Ancuța P, Sonia A (2020) Oil press-cakes and meals valorization through circular economy approaches: a review. NATO Adv Sci Inst Ser E Appl Sci 10:7432. https://doi.org/10.3390/app10217432
Sousa D, Salgado JM, Cambra-López M et al (2022) Degradation of lignocellulosic matrix of oilseed cakes by solid-state fermentation: fungi screening for enzymes production and antioxidants release. J Sci Food Agric 102:1550–1560. https://doi.org/10.1002/jsfa.11490
Olaiya BC, Lawan MM, Olonade KA (2023) Utilization of sawdust composites in construction—a review. SN Appl Sci 5:140. https://doi.org/10.1007/s42452-023-05361-4
Sousa D, Salgado JM, Cambra-López M et al (2023) Bioprocessing of oilseed cakes by fungi consortia: impact of enzymes produced on antioxidants release. J Biotechnol 364:5–12. https://doi.org/10.1016/j.jbiotec.2023.01.008
Su L-H, Zhao S, Jiang S-X et al (2017) Cellulase with high β-glucosidase activity by Penicillium oxalicum under solid state fermentation and its use in hydrolysis of cassava residue. World J Microbiol Biotechnol 33:37. https://doi.org/10.1007/s11274-016-2200-7
Kumar A, Gautam A, Dutt D (2016) Co-cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a cost-effective method to produce cellulases for the hydrolysis of pearl millet stover. Fermentation 2:12. https://doi.org/10.3390/fermentation2020012
Yang K, Qing Y, Yu Q et al (2021) Byproduct feeds: current understanding and future perspectives. Collect FAO Agric 11:207. https://doi.org/10.3390/agriculture11030207
Santos FA, de Carvalho-Gonçalves LCT, Cardoso-Simões AL, de Santos C M (2021) Evaluation of the production of cellulases by Penicillium sp. FSDE15 using corncob and wheat bran as substrates. Bioresource Technol Rep 14:100648. https://doi.org/10.1016/j.biteb.2021.100648
Camassola M, Dillon AJP (2010) Cellulases and xylanases production by Penicillium echinulatum grown on sugar cane bagasse in solid-state fermentation. Appl Biochem Biotechnol 162:1889–1900. https://doi.org/10.1007/s12010-010-8967-3
Camassola M, Dillon AJP (2007) Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. J Appl Microbiol 103:2196–2204. https://doi.org/10.1111/j.1365-2672.2007.03458.x
Tharani D, Ananthasubramanian M (2021) Process intensification in separation and recovery of biogenic volatile fatty acid obtained through acidogenic fermentation of organics-rich substrates. Chem Eng Process - Process Intensif 169:108592. https://doi.org/10.1016/j.cep.2021.108592
Kawaguchi H, Hasunuma T, Ogino C, Kondo A (2016) Bioprocessing of bio-based chemicals produced from lignocellulosic feedstocks. Curr Opin Biotechnol 42:30–39. https://doi.org/10.1016/j.copbio.2016.02.031
Chong G-G, Huang X-J, Di J-H et al (2018) Biodegradation of alkali lignin by a newly isolated Rhodococcus pyridinivorans CCZU-B16. Bioprocess Biosyst Eng 41:501–510. https://doi.org/10.1007/s00449-017-1884-x
Molina G, Abrahão MRE, Pessôa MG et al (2016) Industrial additives obtained through microbial biotechnology: bioflavours and biocolourants. Handbook Microb Bioresource 549–566. https://doi.org/10.1079/9781780645216.0549
Pessôa MG, Vespermann KAC, Paulino BN et al (2019) Newly isolated microorganisms with potential application in biotechnology. Biotechnol Adv 37:319–339. https://doi.org/10.1016/j.biotechadv.2019.01.007
Hadj Saadoun J, Bertani G, Levante A et al (2021) Fermentation of agri-food waste: a promising route for the production of aroma compounds. Foods 10. https://doi.org/10.3390/foods10040707
Berger RG (2015) Biotechnology as a source of natural volatile flavours. Curr Opin Food Sci 1:38–43. https://doi.org/10.1016/j.cofs.2014.09.003
Han L, Feng J, Zhang S et al (2012) Alkali pretreated of wheat straw and its enzymatic hydrolysis. Braz J Microbiol 43:53–61. https://doi.org/10.1590/S1517-83822012000100006
Tavares MP, Morgan T, Gomes RF et al (2021) Secretomic insight into the biomass hydrolysis potential of the phytopathogenic fungus Chrysoporthe cubensis. J Proteom 236:104121. https://doi.org/10.1016/j.jprot.2021.104121
Corrêa TLR, Román EKB, da Silva Cassoli J et al (2022) Secretome analysis of Trichoderma reesei RUT-C30 and Penicillium Oxalicum reveals their synergic potential to deconstruct sugarcane and energy cane biomasses. Microbiol Res 260:127017. https://doi.org/10.1016/j.micres.2022.127017
Huang X, Li D, Wang L-J (2017) Characterization of pectin extracted from sugar beet pulp under different drying conditions. J Food Eng 211:1–6. https://doi.org/10.1016/j.jfoodeng.2017.04.022
da Silva Delabona P, Farinas CS, da Silva Lima DJ, da Cruz Pradella JG (2013) Experimental mixture design as a tool to enhance glycosyl hydrolases production by a new Trichoderma harzianum P49P11 strain cultivated under controlled bioreactor submerged fermentation. Bioresour Technol 132:401–405. https://doi.org/10.1016/j.biortech.2012.11.087
Rai R, Kaur B, Singh S et al (2016) Evaluation of secretome of highly efficient lignocellulolytic Penicillium sp. Dal 5 isolated from rhizosphere of conifers. Bioresour Technol 216:958–967. https://doi.org/10.1016/j.biortech.2016.06.040
Oliveira F, Souza CE, Peclat VROL et al (2017) Optimization of lipase production by Aspergillus ibericus from oil cakes and its application in esterification reactions. Food Bioprod Process 102:268–277. https://doi.org/10.1016/j.fbp.2017.01.007
Jain R, Naik SN (2018) Adding value to the oil cake as a waste from oil processing industry: production of lipase in solid state fermentation. Biocatal Agric Biotechnol 15:181–184. https://doi.org/10.1016/j.bcab.2018.06.010
Acknowledgements
The authors thank Abhishek Agarwal, Laboratory of Functional and Structural Genomics, Centre of New Technologies, University of Warsaw, for helping implement the R script for creating a graph.
Funding
The authors gratefully acknowledge the Foundation for Polish Science’s TEAM-NET program (grant no. POIR.04.04.00-00-14E6/18-00) under the project titled “Fly ash as precursors of functionalized materials for applications in environmental engineering, civil engineering, and agriculture” and IDUB UW microgrants (grant no: BOB-IDUB-622-46_2022) under Excellence Initiative-Research University for University of Warsaw PhD students under action IV.4.1 for supporting the present work.
Author information
Authors and Affiliations
Contributions
Namrata Joshi: conceptualization, methodology, formal analysis, and writing—original draft. Jasneet Grewal: investigation, formal analysis, and writing—review and editing. Robert Stasiuk: methodology and formal analysis. Lukasz Drewniak: writing (review and editing) and funding acquisition. Kumar Pranaw: conceptualization, supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
This article contains no studies with human participants or animals performed by any authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joshi, N., Grewal, J., Stasiuk, R. et al. Unveiling the secretome of Penicillium fuscoglaucum JAM-1 for efficient dual substrate degradation and waste valorization. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05809-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05809-6