Abstract
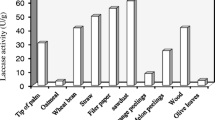
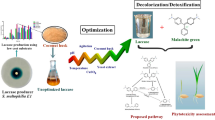
In this study, Bacillus subtilis strain KSK02 consumed waste groundnut shell and Borassus flabellifer fruit husk to produce laccase using solid-state fermentation (SSF) by response surface methodology-Box-Behnken design (BBD-RSM). In order to enhance the biodegradability of groundnut shell waste (GNSW) and Borassus flabellifer fruit husk waste (BFFHW), the alkaline pre-treatment of both of these waste products was explored in this work. The morphological changes induced by the pre-treatment of both the GNSW and the BFFHW were characterized by scanning electron microscopy (SEM). The initial pH, incubation time, and concentrations of GNSW and BFFHW variables were designed for each fermentation condition of the B. subtilis strain KSK02 to enhance laccase production. B. subtilis strain KSK02 was able to produce a maximum production of amylase (423 U/mL) under optimal conditions during the fermentation process. These conditions included GNSW (2 g/L), BFEFBW (1.5 g/L), pH 7.0, and incubation time of 96 h. The maximum 89% decolorization was observed after 24 h of bacterial laccase treatment. The efficacy of the bacterial laccase in removing reactive red-120 (RR-120) from aqueous solutions using Vigna radiata seeds was demonstrated in the phytotoxic test. Molecular docking exhibited the binding affinity score of − 54.62 kcal/mol between the laccase-RR-120 dye complex. This study outlines statistically performed optimization approaches for improving bacterial laccase production, which resulted in a production system that is both high-yielding and cost-effective for a laccase with potential applications in textile color removal.









Similar content being viewed by others
Data availability
The essential data supporting the reported results are contained in this study. All other data are available on request from the corresponding authors.
References
Asha S, Hentry C, Bindhu MR et al (2021) Improved photocatalytic activity for degradation of textile dyeing waste water and thiazine dyes using PbWO4 nanoparticles synthesized by co-precipitation method. Environ Res 200:111721. https://doi.org/10.1016/j.envres.2021.111721
Basumatary S, Kumar KJ, Daimari J et al (2023) Biosynthesis of silver nanoparticles using Antidesma acidum leaf extract: its application in textile organic dye degradation. Environ Nanotechnol Monit Manag 19:100769. https://doi.org/10.1016/j.enmm.2022.100769
Rima SAJ, Paul GK, Islam S et al (2022) Efficacy of Pseudomonas sp. and Bacillus sp. in textile dye degradation: a combined study on molecular identification, growth optimization, and comparative degradation. J Hazard Mater Lett 3:100068. https://doi.org/10.1016/j.hazl.2022.100068
Carney Almroth B, Cartine J, Jönander C et al (2021) Assessing the effects of textile leachates in fish using multiple testing methods: from gene expression to behavior. Ecotoxicol Environ Saf 207:111523. https://doi.org/10.1016/j.ecoenv.2020.111523
Dihom HR, Al-Shaibani MM, Radin Mohamed RMS et al (2022) Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: a critical review. J Water Process Eng 47:102705. https://doi.org/10.1016/j.jwpe.2022.102705
Jorge AMS, Athira KK, Alves MB et al (2023) Textile dyes effluents: a current scenario and the use of aqueous biphasic systems for the recovery of dyes. J Water Process Eng 55:104125. https://doi.org/10.1016/j.jwpe.2023.104125
Al-Tohamy R, Ali SS, Xie R et al (2023) Decolorization of reactive azo dye using novel halotolerant yeast consortium HYC and proposed degradation pathway. Ecotoxicol Environ Saf 263:115258. https://doi.org/10.1016/j.ecoenv.2023.115258
Miao M, Lu Q, Wang X et al (2023) Removal of micro-organic contaminants from wastewater: a critical review of treatment technology. Next Mater 1:100016. https://doi.org/10.1016/j.nxmate.2023.100016
Xuejiao A, Yi C, Ningjian L et al (2023) Green and sustainable approach for decoloration and detoxification of azo dye by bacterial-mediated thermo-halotolerant dye-decolorizing peroxidase (DyP). Process Biochem 130:334–346. https://doi.org/10.1016/j.procbio.2023.03.037
Intisar A, Ramzan A, Hafeez S et al (2023) Adsorptive and photocatalytic degradation potential of porous polymeric materials for removal of pesticides, pharmaceuticals, and dyes-based emerging contaminants from water. Chemosphere 336:139203. https://doi.org/10.1016/j.chemosphere.2023.139203
Lu T, Zhang Q, Yao S (2017) Efficient decolorization of dye-containing wastewater using mycelial pellets formed of marine-derived Aspergillus niger. Chin J Chem Eng 25:330–337. https://doi.org/10.1016/j.cjche.2016.08.010
Oke EA, Ijardar SP (2021) Insights into the separation of metals, dyes and pesticides using ionic liquid based aqueous biphasic systems. J Mol Liq 334:116027. https://doi.org/10.1016/j.molliq.2021.116027
Oke EA, Sharma R, Malek NI, Ijardar SP (2022) Chapter 10 - applications of deep eutectic solvents in remediation of emerging contaminants. In: Pandey A, Tiwari B, Pandey A, Yusup S (eds) Current developments in biotechnology and bioengineering. Elsevier, pp 223–246
Oke EA, Ijardar SP (2023) Partitioning behavior of dyes in ionic liquids induced aqueous biphasic systems of PPG 725 & ammonium salts. Sep Sci Technol 58:2463–2481. https://doi.org/10.1080/01496395.2023.2259600
Oke EA, Ijardar SP (2023) Aqueous biphasic systems composed of alcohol-based deep eutectic solvents and inorganic salts: application in the extraction of dyes with varying hydrophobicity. J Mol Liq 375:121372. https://doi.org/10.1016/j.molliq.2023.121372
Bilińska L, Gmurek M (2021) Novel trends in AOPs for textile wastewater treatment. Enhanced dye by-products removal by catalytic and synergistic actions. Water Resour Ind 26:100160. https://doi.org/10.1016/j.wri.2021.100160
Keskin B, Ersahin ME, Ozgun H, Koyuncu I (2021) Pilot and full-scale applications of membrane processes for textile wastewater treatment: a critical review. J Water Process Eng 42:102172. https://doi.org/10.1016/j.jwpe.2021.102172
Sharma M, Tyagi VV, Chopra K et al (2023) Advancement in solar energy-based technologies for sustainable treatment of textile wastewater: reuse, recovery and current perspectives. J Water Process Eng 56:104241. https://doi.org/10.1016/j.jwpe.2023.104241
Velásquez-Quintero C, Merino-Restrepo A, Hormaza-Anaguano A (2022) Production, extraction, and quantification of laccase obtained from an optimized solid-state fermentation of corncob with white-rot fungi. J Clean Prod 370:133598. https://doi.org/10.1016/j.jclepro.2022.133598
Wiśniewska KM, Twarda-Clapa A, Białkowska AM (2021) Screening of novel laccase producers—isolation and characterization of cold-adapted laccase from Kabatiella bupleuri G3 capable of synthetic dye decolorization. Biomolecules 11:828. https://doi.org/10.3390/biom11060828
Elsayed AM, Mahmoud M, Abdel Karim GSA et al (2023) Purification and biochemical characterization of two laccase isoenzymes isolated from Trichoderma harzianum S7113 and its application for bisphenol A degradation. Microb Cell Fact 22:1. https://doi.org/10.1186/s12934-022-02011-z
Ariaeenejad S, Motamedi E (2023) Improved saccharification of rice straw by removing phenolic compounds using a stable immobilized metagenome-derived laccase on sodium alginate-based hydrogel. Biochem Eng J 198:109021. https://doi.org/10.1016/j.bej.2023.109021
Bhardwaj P, Sharma S, Khatri M et al (2023) Eradication of ibuprofen and diclofenac via in situ synthesized and immobilized bacterial laccase to Cu-based metal organic framework. J Water Process Eng 54:104023. https://doi.org/10.1016/j.jwpe.2023.104023
da Rocha AB, de Aquino SR, de Siqueira VM et al (2023) Shrimp laccase degrades polycyclic aromatic hydrocarbons from an oil spill disaster in Brazil: a tool for marine environmental bioremediation. Mar Pollut Bull 194:115445. https://doi.org/10.1016/j.marpolbul.2023.115445
Jia Y, Huang Q, Zhu L, Pan C (2022) Characterization of a recombinant laccase B from Trametes hirsuta MX2 and its application for decolorization of dyes. Molecules 27:1581. https://doi.org/10.3390/molecules27051581
Liu X, Deng W, Yang Y (2021) Characterization of a novel laccase LAC-Yang1 from white-rot fungus Pleurotus ostreatus strain Yang1 with a strong ability to degrade and detoxify chlorophenols. Molecules 26:473. https://doi.org/10.3390/molecules26020473
Nayak B, Choudhary R (2022) Optimization, purification and characterization of laccase from lignocellulolytic fungi Dichotomopilus funicola NFCCI 4534 and Alternaria padwickii NFCCI 4535. Biocatal Agric Biotechnol 42:102344. https://doi.org/10.1016/j.bcab.2022.102344
Ajayi VA, Lateef A (2023) Biotechnological valorization of agrowastes for circular bioeconomy: melon seed shell, groundnut shell and groundnut peel. Clean Circ Bioecon 4:100039. https://doi.org/10.1016/j.clcb.2023.100039
Balachandran C, Vishali A, Nagendran NA et al (2021) Optimization of protease production from Bacillus halodurans under solid state fermentation using agrowastes. Saudi J Biol Sci 28:4263–4269. https://doi.org/10.1016/j.sjbs.2021.04.069
Chaudhary N, Grover M (2023) Bioindustrial applications of thermostable endoglucanase purified from Trichoderma viride towards the conversion of agrowastes to value-added products. Protein Expr Purif 211:106324. https://doi.org/10.1016/j.pep.2023.106324
Pranay K, Padmadeo SR, Prasad B (2019) Production of amylase from Bacillus subtilis sp. strain KR1 under solid state fermentation on different agrowastes. Biocatal Agric Biotechnol 21:101300. https://doi.org/10.1016/j.bcab.2019.101300
Sathiparan N, Anburuvel A, Selvam VV (2023) Utilization of agro-waste groundnut shell and its derivatives in sustainable construction and building materials – a review. J Build Eng 66:105866. https://doi.org/10.1016/j.jobe.2023.105866
Selvam K, Sudhakar C, Selvankumar T (2021) Activated carbon derived from Borassus flabellifer fruit husk waste for enhanced removal of reactive red 120. Environ Technol Innov 23:101752. https://doi.org/10.1016/j.eti.2021.101752
Leite P, Sousa D, Fernandes H et al (2021) Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr Opin Green Sustain Chem 27:100407. https://doi.org/10.1016/j.cogsc.2020.100407
Teigiserova DA, Bourgine J, Thomsen M (2021) Closing the loop of cereal waste and residues with sustainable technologies: an overview of enzyme production via fungal solid-state fermentation. Sustain Prod Consumpt 27:845–857. https://doi.org/10.1016/j.spc.2021.02.010
Yafetto L, Odamtten GT, Wiafe-Kwagyan M (2023) Valorization of agro-industrial wastes into animal feed through microbial fermentation: a review of the global and Ghanaian case. Heliyon 9:e14814. https://doi.org/10.1016/j.heliyon.2023.e14814
Oluyinka OA, Oke EA, Oyelude EO et al (2022) Recapitulating potential environmental and industrial applications of biomass wastes. J Mater Cycles Waste Manag 24:2089–2107. https://doi.org/10.1007/s10163-022-01473-y
Manojkumar N, Muthukumaran C, Sharmila G (2022) A comprehensive review on the application of response surface methodology for optimization of biodiesel production using different oil sources. J King Saud Univ - Eng Sci 34:198–208. https://doi.org/10.1016/j.jksues.2020.09.012
Vasiee A, Behbahani BA, Yazdi FT, Moradi S (2016) Optimization of the production conditions of the lipase produced by Bacillus cereus from rice flour through Plackett-Burman Design (PBD) and response surface methodology (RSM). Microb Pathog 101:36–43. https://doi.org/10.1016/j.micpath.2016.10.020
Ambika KV, Jamwal A et al (2022) Green bioprocess for degradation of synthetic dyes mixture using consortium of laccase-producing bacteria from Himalayan niches. J Environ Manage 310:114764. https://doi.org/10.1016/j.jenvman.2022.114764
Green MR, Sambrook J, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
de Oliveira RP, dos Santos BV, Costa L et al (2017) Xylanase and β-glucosidase production by Aspergillus fumigatus using commercial and lignocellulosic substrates submitted to chemical pre-treatments. Ind Crops Prod 95:453–459. https://doi.org/10.1016/j.indcrop.2016.10.055
Retamal CA, Thiebaut P, Alves EW (1999) Protein purification from polyacrylamide gels by sonication extraction. Anal Biochem 268:15–20. https://doi.org/10.1006/abio.1998.2977
Atalla MM, Zeinab HK, Eman RH et al (2013) Characterization and kinetic properties of the purified Trematosphaeria mangrovei laccase enzyme. Saudi J Biol Sci 20:373–381. https://doi.org/10.1016/j.sjbs.2013.04.001
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Gaur N, Narasimhulu K, Y P, (2018) Biochemical and kinetic characterization of laccase and manganese peroxidase from novel Klebsiella pneumoniae strains and their application in bioethanol production. RSC Adv 8:15044–15055. https://doi.org/10.1039/C8RA01204K
Kumar A, Sharma KK, Kumar P, Ramchiary N (2015) Laccase isozymes from Ganoderma lucidum MDU-7: isolation, characterization, catalytic properties and differential role during oxidative stress. J Mol Catal B Enzym 113:68–75. https://doi.org/10.1016/j.molcatb.2015.01.010
Sun Y, Liu Z-L, Hu B-Y et al (2021) Purification and characterization of a thermo- and pH-stable laccase from the litter-decomposing fungus Gymnopus luxurians and laccase mediator systems for dye decolorization. Front Microbiol 12:672620. https://doi.org/10.3389/fmicb.2021.672620
Himanshu CS, Singh A et al (2023) Purification and characterization of laccase from Ganoderma lucidum and its application in decolorization of malachite green dye. Bioresour Technol Rep 21:101368. https://doi.org/10.1016/j.biteb.2023.101368
Yadav A, Yadav P, Kumar Singh A et al (2021) Decolourisation of textile dye by laccase: process evaluation and assessment of its degradation bioproducts. Biores Technol 340:125591. https://doi.org/10.1016/j.biortech.2021.125591
Bergey DH (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams & Wilkins, Baltimore
Kumar BS (2018) Practical microbiology: a laboratory manual. Panima Publishing Corporation
Sondhi S, Saini K (2019) Response surface based optimization of laccase production from Bacillus sp. MSK-01 using fruit juice waste as an effective substrate. Heliyon 5:e01718. https://doi.org/10.1016/j.heliyon.2019.e01718
Sharma S, Sharma V, Kuila A (2016) Cellulase production using natural medium and its application on enzymatic hydrolysis of thermo chemically pretreated biomass. 3 Biotech 6:139. https://doi.org/10.1007/s13205-016-0465-z
Tang W, Huang C, Ling Z, He Y-C (2023) Enhancing cellulosic digestibility of wheat straw by adding sodium lignosulfonate and sodium hydroxide to hydrothermal pretreatment. Biores Technol 379:129058. https://doi.org/10.1016/j.biortech.2023.129058
Pant S, Ritika KA et al (2021) NaOH pretreatment and enzymatic hydrolysis of Brassica juncea using mixture of cellulases. Environ Technol Innov 21:101324. https://doi.org/10.1016/j.eti.2020.101324
Muthukumarasamy NP, Jackson B, Joseph Raj A, Sevanan M (2015) Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate. Biochem Res Int 2015:765190. https://doi.org/10.1155/2015/765190
Al-sareji OJ, Meiczinger M, Al-Juboori RA et al (2023) Efficient removal of pharmaceutical contaminants from water and wastewater using immobilized laccase on activated carbon derived from pomegranate peels. Sci Rep 13:11933. https://doi.org/10.1038/s41598-023-38821-3
Afreen S, Anwer R, Singh RK, Fatma T (2018) Extracellular laccase production and its optimization from Arthrospira maxima catalyzed decolorization of synthetic dyes. Saudi J Biol Sci 25:1446–1453. https://doi.org/10.1016/j.sjbs.2016.01.015
Reda FM, Hassan NS, El-Moghazy A-N (2018) Decolorization of synthetic dyes by free and immobilized laccases from newly isolated strain Brevibacterium halotolerans N11 (KY883983). Biocatal Agric Biotechnol 15:138–145. https://doi.org/10.1016/j.bcab.2018.05.020
Khan SI, Sahinkaya M, Colak DN et al (2024) Production and characterization of novel thermostable CotA-laccase from Bacillus altitudinis SL7 and its application for lignin degradation. Enzyme Microb Technol 172:110329. https://doi.org/10.1016/j.enzmictec.2023.110329
Sridharan R, Krishnaswamy V, Kumar PS et al (2021) Analysis and effective separation of toxic pollutants from water resources using MBBR: pathway prediction using alkaliphilic P. mendocina. Sci Total Environ 797:149135. https://doi.org/10.1016/j.scitotenv.2021.149135
Author information
Authors and Affiliations
Contributions
Kandasamy Selvam—supervision, conceptualization, and writing; Chinnappan Sudhakar—methodology, resources, visualization, and writing; Arunagiri Ragu Prasath—investigation, methodology, and writing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selvam, K., Sudhakar, C. & Prasath, A.R. Optimization of laccase production from Bacillus subtilis strain KSK02 utilizing bi-substrates and their reactive red-120 dye degradation potential. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05365-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05365-z