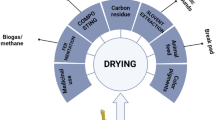
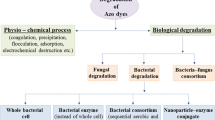
Abstract
The textile industries discharge around 10–15% dyes into effluents, posing threat to human health and environment. White-rot fungi are promising candidates for elimination of variety of dyes, owing to their potential for degradation and mineralization of a broad spectrum of highly toxic, recalcitrant, and organic pollutants. In this study, different species of Pleurotus were evaluated for ligninolytic enzymes production using paddy straw, sugarcane bagasse and wheat straw for 7, 14, 21 and 28 days of incubation, respectively. Among substrates, paddy straw exhibited maximum activity of laccase (Lac) (45968.9 ± 347.2 U/g) and manganese peroxidase (MnP) (16312.1 ± 99.9 U/g) from P. ostreatus D-66 after 28 days, however the lignin peroxidase (LiP) (10823.2 ± 94.5 U/g) activity from P. pulmonarius D-79 following 21 days. Purified Lac, MnP and LiP from P. ostreatus D-66 and P. pulmonarius D-79 showed 68.8, 44.1 and 22.8- fold increase with yield of 57.3%, 36.7% and 30.4%, respectively. These enzymes were stable over wide range of pH (3.5 to 7.5) and temperatures (30 to 50 °C) with optimum pH 4.5–5.0 and temperature 30 °C. Among synthetic dyes, the maximum decolorisation (96.9%) was achieved in Remazol brilliant blue R revealed with Lac from P. ostreatus D-66, while in P. pulmonarius D-79 (88.8%) with malachite green and decolorised dye with optimal pH 4.0–6.0 and temperature 30 °C. The findings demonstrated that paddy straw could be an attractive substrate for Pleurotus spp. to produce ligninolytic enzymes that, after purification, can be exploited effectively for degradation of synthetic dyes from textile effluents.
Graphical abstract








Similar content being viewed by others
Data availability
“Not applicable”.
References
Bhardwaj N, Agrawal K, Kumar B, Verma P (2021) Role of enzymes in deconstruction of waste biomass for sustainable generation of value-added products. Bioprospecting of Enzymes in Industry, Healthcare and Sustainable Environment. Springer, ., pp 219–250
Illuri R, Kumar M, Eyini M, Veeramanikandan V, Almaary KS, Elbadawi YB, Biraqdar MA, Balaji P (2021) Production, partial purification and characterization of ligninolytic enzymes from selected basidiomycetes mushroom fungi. Saudi J Biol Sci 28:7207–7218
Karp SG, Faraco V, Amore A, Birolo L, Giangrande C, Soccol VT, Pandey A, Soccol CR (2012) Characterization of laccase isoforms produced by Pleurotus ostreatus in solid state fermentation of sugarcane bagasse. Bioresour Technol 114:735–739
Sharma V, Tsai ML, Nargotra P, Chen CW, Sun PP, Singhania RR, Patel AK, Dong CD (2023) Journey of lignin from a roadblock to bridge for lignocellulose biorefineries: A comprehensive review. Sci Total Environ 861:160560
Xu X, Xu Z, Shi S, Lin M (2017) Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresour Technol 241:415–423
Hiloidhari M, Vijay V, Banerjee R, Baruah DC, Rao AB (2021) Energy-carbon-water footprint of sugarcane bioenergy: A district-level life cycle assessment in the state of Maharashtra, India. Renew Sustain Energy Rev 151:111583
Akyuz M, Şule İ, Kirkbag S (2022) Nutrient Content of Pleurotus pulmonarius (Fr.) Quel. Grown on Some Local Lignocellulosic Wastes. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Derg 25:25–30
Dias da Silva S, Carvalho dos Santos E, Cordeiro Mendes, da Silva W, da Silva Alves, Magalhães A, Perrone Barbosa EE, Simas Teixeira MF, Pereira JO, da Mota AJ (2021) Draft Genome Sequence of the Wild Edible Mushroom Pleurotusostreatoroseus DPUA 1720. Microbiol Resour Announc 10:840–20
Kues U, Ruhl M (2011) Multiple multi-copper oxidase gene families in basidiomycetes-what for? Curr Genomics 12:72–94
Eichlerová I, Baldrian P (2020) Ligninolytic enzyme production and decolorization capacity of synthetic dyes by saprotrophic white rot, brown rot, and litter decomposing basidiomycetes. J Fungi 6:301
Mishra S, Lin Z, Pang S, Zhang W, Bhatt P, Chen S (2021) Recent advanced technologies for the characterization of xenobiotic-degrading microorganisms and microbial communities. Front Bioeng Biotechnol 9:632059
Di Blasi C, Signorelli G, Di Russo C, Rea G (1999) Product distribution from pyrolysis of wood and agricultural residues. Ind Eng Chem Res 38(6):2216–2224
TAPPI (2011) Acid-soluble lignin in Wood and Pulp. T222 Om-02 1–7
Childs RE, Bardsley WG (1975) The steady-state kinetics of peroxidase with 2, 2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J 145:93–103
Martínez MJ, Ruiz-Dueñas FJ, Guillén F, Martínez ÁT (1996) Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem 237:424–432
Tien M, Kirk TK (1984) Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci 81:2280–2284
Andrew SM, Titus JA, Zumstein L (2001) Dialysis and concentration of protein solutions. Curr Protoc Toxicol 10:A-3H
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 5259:680–685. https://doi.org/10.1038/227680a0
Zhuo R, Zhang J, Yu H, Ma F, Zhang X (2019) The roles of Pleurotus ostreatus HAUCC 162 laccase isoenzymes in decolorization of synthetic dyes and the transformation pathways. Chemosphere 234:733–745
Lallawmsanga, Leo VV, Passari AK, Muniraj IK, Uthandi S, Hashem A, Abd Allah EF, Alqarawi AA, Singh BP (2019) Elevated levels of laccase synthesis by Pleurotus pulmonarius BPSM10 and its potential as a dye decolorizing agent. Saudi J Biol Sci 26:464–468
Tavares MF, Avelino KV, Araújo NL, Marim RA, Linde GA, Colauto NB, do Valle, J. S. (2020) Decolorization of azo and anthraquinone dyes by crude laccase produced by Lentinuscrinitus in solid state cultivation. Braz J Microbiol 51:99–106
Ezike TC, Ezugwu AL, Udeh JO, Eze SOO, Chilaka FC (2020) Purification and characterisation of new laccase from Trametespolyzona WRF03. Biotechnol Rep 28:e00566
Ayed L, Chaieb K, Cheref A, Bakhrouf A (2010) Biodegradation and decolorization of triphenylmethane dyes by Staphylococcus epidermidis. Desalination 260:137–146
Geng X, Xie X, Liang Y, Li Z, Yang K, Tao J, Zhang H, Wang Z (2021) Facile fabrication of a novel copper nanozyme for efficient dye degradation. ACS Omega 6:6284–6291
Mohajer-Moghari F, SeifpanahiShabani K, Karamouzian M (2019) Pre-treatment of toxic element and cationic dye onto natural biomass: characterization and optimization. J Min Environ 10:649–658
Brugnari T, Braga DM, dos Santos CSA, Torres BHC, Modkovski TA, Haminiuk CWI, Maciel GM (2021) Laccases as green and versatile biocatalysts: from lab to enzyme market—an overview. Bioresour Bioprocess 8:1–29
Ćilerdžić J, Galić M, Stajić M (2022) From pomiculture waste to biotechnological raw material: efficient transformation using ligninosomes and cellulosomes from Pleurotus spp. Bioresour Bioprocess 9:1–11
Vilar DS, Fernandes CD, Nascimento VR, Torres NH, Leite MS, Bharagava RN, Bilal M, Salazar-Banda GR, Eguiluz KIB, Ferreira LFR (2021) Hyper-production optimization of fungal oxidative green enzymes using citrus low-cost byproduct. J Environ Chem Eng 9:105013
Ergun SO, Urek RO (2017) Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Ann Agrar 15:273–277
Patel H, Gupte A, Gupte S (2009) Effect of different culture conditions and inducers on production of laccase by a basidiomycete fungal isolate Pleurotus ostreatus HP-1 under solid state fermentation. BioResources 4:268–284
Gorai B, Sharma R (2018) Effect of Different Substrates on Yield Potential of Pleurotus spp. in West Bengal. Int J Curr Microbiol App Sci 7:2162–2169
Thiribhuvanamala G, Kalaiselvi G, Parthasarathy S, Anusha B (2017) Induction of lignolytic enzyme activities in different agro residues by the white rot fungi, Pleurotus sajar-caju. Int J Chem Stud 5:89–94
Kumar GN, Srikumar K (2011) Thermophilic laccase from xerophyte species Opuntia vulgaris. Biomed Chromatogr 25:707–711
Fakoussa RÁ, Hofrichter M (1999) Biotechnology and microbiology of coal degradation. Appl Microbiol Biotechnol 52:25–40
Rothschild N, Novotny´ C, Sˇasˇek V, Dosoretz CG (2002) Ligninolytic enzymes of the fungus Irpexlacteus (Polyporustulipiferae) isolation and characterization of lignin peroxidase. Enzyme Microb Technol 31:627–633
Yang J, Yuan H, Wang H, Chen W (2005) Purification and characterization of lignin peroxidases from Penicillium decumbens P6. World J Microbiol Biotechnol 21:435–440
Heinfling A, Martinez MJ, Martinez AT, Bergbauer M, Szewzyk U (1998) Purification and characterization of peroxidases from the dye-decolorizing fungus Bjerkandera adusta. FEMS Microbiol Lett 165:43–50
Bermek H, Yazıcı H, Öztürk H, Tamerler C, Jung H, Li K, Brown KM, Ding H, Xu F (2004) Purification and characterization of manganese peroxidase from wood-degrading fungus Trichophyton rubrum LSK-27. Enzyme Microbiol Technol 35:87–92
Yehia RS (2014) Aflatoxin detoxification by manganese peroxidase purified from Pleurotus ostreatus. Braz J Microbiol 45:127–134
Sayyed RZ, Bhamare HM, Marraiki N, Elgorban AM, Syed A, El-Enshasy HA, Dailin DJ (2020) Tree bark scrape fungus: A potential source of laccase for application in bioremediation of non-textile dyes. PLoS ONE 15:e0229968
Backes E, Kato CG, da Silva TB, Uber TM, Pasquarelli DL, Bracht A, Peralta RM (2022) Production of fungal laccase on pineapple waste and application in detoxification of malachite green. J Environ Sci Health Part B 57:90–101
Baldrian P (2006) Fungal laccases—Occurrence and properties. FEMS Microbiol Rev 30(2):215–242. https://doi.org/10.1111/j.1574-4976.2005.00010.x
Halaburgi VM, Sharma S, Sinha M, Singh TP, Karegoudar TB (2011) Purification and characterization of a thermostable laccase from the ascomycetes Cladosporium cladosporioides and its applications. Process Biochem 46:1146–1152
Alam MZ, Mansor MF, Jalal KCA (2009) Optimization of lignin peroxidase production and stability by Phanerochaete chrysosporium using sewage-treatment-plant sludge as substrate in a stirred-tank bioreactor. J Ind Microbiol Biotechnol 36:757–764
Ürek RÖ, Pazarlioğlu NK (2004) Purification and partial characterization of manganese peroxidase from immobilized Phanerochaete chrysosporium. Process Biochem 39:2061–2068
Ali EAM, AbdEllatif S, Abdel Razik E (2020) Production, purification, characterization and immobilization of laccase from Phoma betae and its application in synthetic dyes decolorization. Egypt J Bot 60:301–312
Aslam S, Asgher M (2011) Partial purification and characterization of ligninolytic enzymes produced by Pleurotus ostreatus during solid state fermentation. Afr J Biotechnol 10:17875–17883
Nadeem A, Baig S, Sheikh N (2014) Mycotechnological production of laccase by Pleurotus ostreatus-P1 and its inhibition study. J Anim Plant Sci 24:492–502
Baldrian P (2004) Purification and characterization of laccase from the white-rot fungus Daedalea quercina and decolorization of synthetic dyes by the enzyme. Appl Microbiol Biotechnol 63:560–563
Zhang H, Zhang J, Zhang X, Geng A (2018) Purification and characterization of a novel manganese peroxidase from white-rot fungus Cerrena unicolor BBP6 and its application in dye decolorization and denim bleaching. Process Biochem 66:222–229
Tinoco R, Pickard MA, Vazquez-Duhalt R (2001) Kinetic differences of purified laccases from six Pleurotus ostreatus strains. Lett Appl Microbiol 32:331–335
Zeng X, Cai Y, Liao X, Zeng X, Li W, Zhang D (2011) Decolorization of synthetic dyes by crude laccase from a newly isolated Trametes trogii strain cultivated on solid agro-industrial residue. J Hazard Mater 187:517–525
Radha KV, Regupathi I, Arunagiri A, Murugesan T (2005) Decolorization studies of synthetic dyes using Phanerochaete chrysosporium and their kinetics. Process Biochem 40:3337–3345
Soares GM, de Amorim MP, Costa-Ferreira M (2001) Use of laccase together with redox mediators to decolourize Remazol Brilliant Blue R. J Biotechnol 89:123–129
Bibi I, Bhatti HN (2012) Enhanced biodecolorization of reactive dyes by basidiomycetes under static conditions. Appl Biochem Biotechnol 166:2078–2090
Ha H-C (2021) Production of manganese peroxidase from Pleurotus ostreatus using a rotary draft tube bioreactor (RTB) and characterization of its activity. J Mushroom 19:316–321
Cruz YW, Vieira YA, Vilar DS, Torres NH, Aguiar MM, Cavalcanti EB, Américo-Pinheiro JH, Soriano RN, Bharagava RN, Lima ÁS, Ferreira LF (2020) Pulp wash: a new source for production of ligninolytic enzymes and biomass and its toxicological evaluation after biological treatment. Environ Technol 41:1837–1847
Fernandes CD, Nascimento VRS, Meneses DB, Vilar DS, Torres NH, Leite MS et al (2020) Fungal biosynthesis of lignin-modifying enzymes from pulp wash and Luffa cylindrica for azo dye RB5 biodecolorization using modeling by response surface methodology and artificial neural network. J Hazard Mater 399:123–194
Sartori SB, Ferreira LFR, Messias TG, Souza GD, Pompeu GB, Monteiro RTR (2015) Pleurotus biomass production on vinasse and its potential use for aquaculture feed. Mycology 6:28–34
Junior JA, Vieira YA, Cruz IA, da Silva VD, Aguiar MM, Torres NH et al (2020) Sequential degradation of raw vinasse by a laccase enzyme producing fungus Pleurotus sajor-caju and its ATPS purification. Biotechnol Rep 25:4–11
Ferreira LFR, Torres NH, de Armas RD, Fernandes CD, da Silva Vilar D, Aguiar MM, Pompeu GB, Monteiro RTR, Iqbal HM, Bilal M, Bharagava RN (2020) Fungal lignin-modifying enzymes induced by vinasse mycodegradation and its relationship with oxidative stress. Biocatal Agric Biotechnol 27:101691
Katia MCM, Lucina CAC, Rubio OM, Luiz HR, Mercia HS (2006) Biodegradation of reactive textile dyes by basidiomycetous fungi from Brazilian ecosystems. Braz J Microbiol 37:481–487
Ramírez-Montoya LA, Hernández-Montoya V, Montes-Morán MA, Jáuregui-Rincón J, Cervantes FJ (2015) Decolorization of dyes with different molecular properties using free and immobilized laccases from Trametes versicolor. J Mol Liq 212:30–37
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26:201–221
Hadibarata T, Yusoff ARM, Kristanti RA (2012) Decolorization and metabolism of anthraquinone-type dye by laccase of white-rot fungi Polyporus sp. S133. Water Air Soil Pollut 223:933–941
Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2010) Transformation pathway of Remazol Brilliant Blue R by immobilized laccase. Bioresour Technol 101:8509–8514
Glazunova OA, Trushkin NA, Moiseenko KV, Filimonov IS, Fedorova TV (2018) Catalytic efficiency of basidiomycete laccases: redox potential versus substrate-binding pocket structure. Catalysts 8:152
Moldes D, Lorenzo M, Sanromán MÁ (2004) Degradation or polymerisation of Phenol Red dye depending to the catalyst system used. Process Biochem 39:1811–1815
Trevizani JLB, Nagalli A, Passig FH, de Carvalho KQ, Schiavon GJ, de Lima Model AN (2018) Influence of pH and concentration on the decolorization and degradation of BR red azo dye by ozonization. Acta Sci Technol 40:e35436–e35436
Erden E, Kaymaz Y, Pazarlioglu NK (2011) Biosorption kinetics of a direct azo dye Sirius Blue K-CFN by Trametes versicolor. Electron J Biotechnol 14:1-e10
Pype R, Flahaut S, Debaste F (2019) On the importance of mechanisms analysis in the degradation of micropollutants by laccases: The case of Remazol Brilliant Blue R. Environ Technol Innov 14:100324
Shazia E, Safia A (2011) Comparison of dye decolorization efficiency of indigenous fungal isolates. Afr J Biotechnol 10:3399–3411
Machado KMG, Matheus DR (2006) Biodegradation of Remazol brilliant blue R by ligninolytic enzymatic complex produced by Pleurotus ostreatus. Braz J Microbiol 37:468–473
Deveci T, Unyayar A, Mazmanci MA (2004) Production of Remazol Brilliant Blue R decolourising oxygenase from the culture filtrate of Funalia trogii ATCC 200800. J Mol Catal B: Enzym 30:25–32
Murugesan K, Dhamija A, Nam IH, Kim YM, Chang YS (2007) Decolourization of reactive black 5 by laccase: Optimization by response surface methodology. Dyes Pigm 75:176–184
Acknowledgements
Authors are thankful to The Director, ICAR-National Bureau of Agriculturally Important Microorganisms (NBAIM) for providing necessary facilities.
Funding
Authors received no specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
ASR and PC wrote first draft of this manuscript. HC, VM, NK supported for performing experiments in their laboratory. NS, KP and NK design the complete experiment and supervise during this research.
Corresponding author
Ethics declarations
Ethical approval
“Not applicable”.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajavat, A.S., Shrivastava, N., Choudhary, P. et al. Valorization of agro-residues for production of ligninolytic enzymes from Pleurotus spp. and their deployment in dye decolorisation. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05004-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05004-z