Abstract
Bio-valorization of various biomasses provides a sustainable promising approach for the eco-friendly production of variable value-added products. Herein, the current study devoted to the enzymatic valorization of two widely available biomasses, namely, maize stalks and waste paper. The cellulytic and hemicellulytic-rich cocktail was produced through the fermentation of rice straw by a locally isolated fungal strain Aspergillus terreus. The potential applicability of the produced cocktail for the enzymatic hydrolysis of the polysaccharide constituents of maize stalks was evaluated under various strategies. The reported results indicated that the microwave pretreatment of the biomass yielding a water-soluble hydrolyzate rich in cellobiose and xylobiose, sustained by thin layer (TLC) and high-performance liquid chromatographic (HPLC) measurements, in addition to phenolic compounds. Moreover, the enzymatic hydrolysis of the extracted hemicellulosic fraction from maize stalks was rich in xylooligosaccharides and phenolic compounds higher than that released from the hydrolysis of commercial xylan. The estimated antioxidant activity of the resulted hydrolyzate was also monitored by the scavenging of 1,1-diphenyl-2-picrylhydrazyl free radical spectrophotometrically at 515 nm. Moreover, the potential applicability of the produced enzymatic cocktail was examined for the bio-deinking of waste paper. The physical, chemical, and surface morphological characteristics of the treated paper sample was compared to a blank one regarding the whiteness index, ash content, scanning electron microscopy (SEM), energy dispersive X-ray (EDX), and Fourier transform infrared spectroscopy (FTIR). On the base of the estimated results, the produced enzymatic cocktail possessed efficient dislodgement ability for the printed ink from the paper surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Enzymes are highly specific biocatalysts produced by all domains of life for promoting the bioconversion of a targeted substrate. In the last two decades, the industrial production of enzymes attracted a great scope based on their extensive exploitation in various industrial processes in which the microbial sources are commercially favorable [1]. Cellulytic enzymes are common hydrolytic enzymes that capable to catalyze the hydrolysis of cellulose, β-1,4-glycosidic linked glucose chains. They are classified into endoglucanases that act randomly within the chains, cellobiohydrolases that are capable for the release of cellobiose units from both reducing and non-reducing ends of the chains, in addition to β-glucosidases that hydrolyze the cellobiose moiety releasing two glucose subunits. Cellulases are now being applied in various industrial operations including oil extraction, detergent, textile, pulp, and paper industry [2,3,4,5]. In addition, various enzymes are capable for the hydrolysis of other polysaccharide fractions including xylan, a branched polysaccharide of β-1,4-glycosidic linked xylose backbone decorated with various moieties in the side chain including L-arabinose, acetic acid, D-glucuronic, and methyl-glucuronic acid [6], in which the endoxylanases hydrolyze the glycosidic bonds within the xylan backbone with the releasing of the corresponding oligosaccharides. Xylan-hydrolyzing enzymes find wide industrial applications including clarification of juice [7], ethanol production [8], animal feed [9], paper bleaching [10], crop [11], and detergent industry [12].
Lignocellulosic biomasses are renewable natural resources rich in cellulose (35–50%) and hemicellulose (20–35%), a branched hetero-polysaccharide composed mainly of xylose with the distribution of other sugar moieties including mannose, arabionose, and galactose, in addition to lignin fraction (5–30%) [13]. According to their unique composition, lignocellulosic biomasses are attractive sources for the eco-friendly production of value-added products such as sugars and furfurals [14], phenolic compounds [15], and various oligosaccharides [16]. The cellulosic fractions of lignocellulosic biomasses are natural renewal bio-source that used for the production of various biomedical biomaterials [17] in addition to the economic production of cello-oligosaccharides, linear oligomers of 2–6-glucose subunits with a promising prebiotic activity [18]. In addition, the hemicellulosic fraction is considered as a source for various oligosaccharides including xylo-, manno-, and arabino-oligosaccharide that have been considered as non-digestible food components with prebiotic activities [19, 20]. Moreover, such oligosaccharides possessed antioxidant [21, 22], anti-inflammatory [23], and antitumor activities [24]. Natural antioxidants have the ability to overcome cellular deleterious effects of free radicals and consequently reduce cell destruction, aiding in the treatment of various diseases including cancer, cardiovascular disorders, and diabetes [25, 26].
Utilization of lignocellulosic biomasses in an eco-friendly manner for the production of various valuable products has indicated the much-needed impetus in this regard [27]. Enzymatic hydrolysis has been pointed as the method with the least environmental impacts, but the high complicated structure of lignocellulosic biomasses represents a major obstacle against the application of enzymes. Therefore, pretreatment of the biomass represents a crucial step for efficient enzymatic bioconversion of lignocellulosic biomasses. Various chemical and physical strategies including the use of acids, alkalis, deep eutectic solvents, and supercritical fluids in addition to the application of ultrasound and microwave pretreatment represents suitable solutions for reducing the high recalcitrance of lignocellulosic biomasses [28]. Microwave processing satisfies many requirements of green chemistry [29]. It has been considered as an efficient alternative method for conventional heating applied in biomass conversion via the uniform transfer of heat energy within the sample matrix facilitating its utilization with a protective manner for its constitutive components [30,31,32].
In addition to lignocellulosic biomasses, waste paper is an alternative valuable fiber source for paper-making industry. The recycling of waste paper provides a great protective impact to the environment since the forest tree pulps are the global main source for paper manufacturing; 7.5 acres trees are consumed for the production of 700 tons of raw paper [33]. Deinking of waste paper is the outmost important step in waste paper recycling, but unfortunately, the traditional applied technique includes the application of a large number of hazardous chemicals. Therefore, enzymatic pretreatment has attracted the research focus as they are energy efficient and required mild operating conditions with lower environmental impact. Enzymes are capable to modify the paper surface, loosening the interaction between the ink particles and paper surface which stimulate their discharge during washing or floatation steps [33,34,35,36].
Herein, rice straw was used for the economic production of cellulase and xylanase-rich cocktail under solid-state fermentation conditions using the isolated fungus Aspergillus terreus. The produced cocktail was explored for the enzymatic hydrolysis of maize stalks polysaccharide constituent and production of oligosaccharides. The influence of microwave pretreatment of maize stalks on its enzymatic hydrolysis had been evaluated compared to the alkaline pretreatment. Additionally, both the antioxidant activity and phenolic content of the produced hydrolyzates were estimated. Finally, the efficiency of the produced enzymatic cocktail for the bio-deinking of waste paper was tested.
2 Materials and methods
2.1 Materials
All the applied reagents and chemicals were of analytical or HPLC grade. Standard glucose, cellobiose, or carboxymethyl cellulose (CMC) was purchased from Sigma-Aldrich, Saint Louis, USA. Beech wood xylan was produced from SERVA, Heidelberg, Germany. Dinitrosalicylic acid (DNS) (Panreac, Barcelona, Spain) was applied for assaying of the reducing sugars. Xylose, xylobiose, and xylotrise were obtained from Megazyme, Wicklow, Ireland. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical was purchased from Sigma-Aldrich, Saint Louis, USA. Thin layer chromatographic studies were performed on silica gel plates (Merck, Darmstadt, Germany).
2.2 Enzyme production
2.2.1 Microorganism and culture conditions
The fungal strain Aspergillus terreus (Accession no. MN368221) was applied for the production of cellulytic and hemicellulytic-rich enzymatic cocktail through solid-state fermentation of rice straw. Briefly, a production media containing 3.75 g of the moistened rice straw (1:3 ratio biomass to moistening solution composed of (g/L) (NH4)2SO4; 10, KH2PO4; 2, CaCl2; 0.3, MgSO4.7H2O; 0.3 and adjusted to pH 7) was used. The production media was inoculated with 2 mL of spore suspension and incubated at 30 °C for 8 days [37].
At the end of incubation period, the production media was extracted with 50 mL distilled water under shaking at 150 rpm (30 °C) for 1 h and centrifuged for 10 min at 5000 rpm (4 °C). The clear supernatant was precipitated using ethanol, where the 70% precipitated fraction was applied in further experiments.
2.2.2 Enzymatic activity and protein content
The activities of the extracellular cellulytic and hemicellulytic enzymes in the cell free supernatant were individually assayed applying CMC and xylan in a reaction mixture containing 500 µL of the corresponding substrate (1.0% dissolved in 0.05 M acetate buffer solution at pH 5) and 500 µL of the raw enzyme solution followed by incubation at 50 °C for 30 min. The released reducing sugars were estimated immediately according to DNS method [38]. One unit of the enzyme was expressed as the amount of enzyme that capable to release 1 μmol of reducing sugar per minute using glucose and xylose as standards. Contrary, the protein content in the cell free production medium was spectrophotometrically assayed as described by Lowry et al., [39] where bovine serum albumin was used as standard.
2.3 Hydrolytic activity of the enzyme cocktail
Herein, the cellulytic and hemicellulytic activity of the enzyme cocktail was explored using p-nitrophenyl-β-D-glucopyranoside (synthetic glucosidase substrate) and p-nitrophenyl-β-D-xylopyranoside (synthetic exo-xylanase substrate) following the method described by Rustiguel et al., [40] in addition to traditional CMC and xylan substrates.
The hydrolyzates resulted from the enzymatic hydrolysis of CMC and xylan were analyzed on silica gel plate using a mixture of propanol:water:ammonia (70: 20: 10 v/v) as a mobile phase and diphenyl amine-aniline as a spraying reagent [41].
2.4 Hydrolysis of maize stalks constitutive polysaccharides
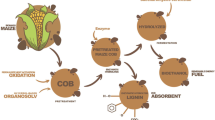
The potential applicability of the produced cocktail for the enzymatic hydrolysis of the polysaccharide constituents of maize stalks was evaluated according to the schematic diagram represented in Fig. 1.
2.4.1 Hydrolysis conditions
Direct hydrolysis
The enzymatic hydrolysis of the un-treated maize stalks was performed according to Zafar et al. [42] with some modification in which the treatment of the waste sample (one gram suspended in 10 mL of 0.05 M acetate buffer at pH 5) was carried out with 10 mL of the enzyme cocktail and incubated at 40 °C for different time intervals (2–24 h). By the end of each specified interval, the enzymatic reaction was terminated by boiling for 10 min and centrifuged for 5 min at 4000 rpm at 25 °C. The enzymatically released reducing sugars were quantified with DNS method [38]. The hydrolysis percentage was estimated as follows:
where S was the amount of the released reducing sugars after specific time and T was the amount of maize stalks added in the reaction.
Microwave pretreatment
Microwave pretreatment had been reported as efficient treatment that assisted the autohydolysis of maize biomasses [43]. In the current study, 10 g of maize stalks were suspended in 100 mL of 0.2 M acetate buffer at pH 5 followed by microwave treatment for 1 min. The waste suspension was filtered with a piece of cotton mesh, washed with distilled water, and dried overnight at 50 °C. Recovery percentage of the treated waste sample was calculated as follow:
where As was the remaining amount after treatment and At was the initial amount of the waste.
The treated sample was hydrolyzed typically as that previously described for the un-treated one.
Alkaline pretreatment
Alkaline treatment of maize stalks was performed following the reported method by Chen and Anderson, [44] with some modifications. Ten grams of maize stalks were suspended in 100 mL NaOH solution (4%), incubated for 24 h at room temperature. The treated sample was filtered, and the solid residue was washed several times with distilled water and dried overnight at 50 °C. The dried residue was hydrolyzed as described for the un-treated maize stalks, while the filtrate was used for the preparation of the hemicellulose fraction.
2.4.2 Extraction of maize stalks hemicellulose fraction
The pH of the filtrate obtained from the above alkaline treatment process was adjusted to 5 using concentrated HCl solution, mixed with double the volume by ethanol (95%), and left to stand for 24 h at room temperature. The collected hemicellulose precipitate, was washed several times with ethanol, and was dried in air [44]. The moisture content of the produced fraction and its water solubility were measured as described by Cano-Chauca et al. [45].
Enzymatic hydrolysis of the resulted hemicellulosic fraction was performed using 2% (w/v) of hemicellulose suspended in 0.05 M acetate buffer at pH 5 in compare to commercial xylan.
2.4.3 Analysis of the resulted hydrolyzate
Thin layer chromatographic analysis
All the resulted hydrolyzates (50 μL) were subjected to TLC analysis as described previously in Section 2.3.
High-performance liquid chromatographic analysis
The resulted hydrolyzate solutions were analyzed with HPLC using glucose, xylose, arabinose, cellobiose, xylobiose, and xylotriose as standards using Agilent Technology 1100 series liquid chromatograph accompanied with a refractive index detector. Shim-pack SCR-101N column was applied where ultrapure water was used as a mobile phase at flow rate 0.7 mL/min.
Fourier transforms infrared spectroscopy
The nature of the active functional groups and chemical bonds in the produced hemicellulose fraction and all of the produced hydrolyzates were examined in compare to the commercial xylan. The dried hydrolyzate was well grinded to fine powder then analyzed using Vertex 80v, Bruker Fourier transform infrared spectrophotometer.
Total phenolic content
The total phenolic contents in the dried hydrolyzate samples were estimated following the procedure described by Ainsworth and Gillespie [46]. The sample solution was mixed with Folin-Ciocalteu’s reagent (1 mL of 10% solution) and 5 mL of 7% Na2CO3 and then left to stand in dark for 2 h. The absorbance of the formed blue colored species was monitored at 760 nm using gallic acid as a standard. The total phenolic content was expressed in mg gallic acid equivalent (GAE)/g dry sample.
2.4.4 Micro-structural analysis of the treated maize stalks
The surface morphology of the treated samples was investigated using high resolution field emission scanning electron microscope (Quanta 250, HRFEG, Czech) in which the gold-coated dried samples were viewed at 20 kV accelerating voltage and 800 magnification power.
2.4.5 Antioxidant activity
The scavenging activity of DPPH radical was examined for all hydrolyzate samples as previously reported by Brand-Williams et al. [47]. Briefly, the hydrolyzate solution (100 μL of 10% (w/v) was mixed with the DPPH methanolic solution (1.1 × 10−4 molL−1) and left for 30 min in dark. The diminishing of the DPPH absorbance was monitored spectrophotometrically at 515 nm, where the results were expressed in μg Trolox equivalents (TE)/mg of the dry sample.
2.5 Bio-deinking of office waste paper
2.5.1 Bio-deinking process
The waste paper samples were collected from different printing offices located in Giza, Egypt, in which laser jet printer with black laser toner was used. Waste paper samples were handled as previously described in details by Hasanin et al. [35]. The potential applicability of the enzymatic cocktail in the deinking of waste papers was performed by soaking of the paper sample (1.0 g) in 0.05 M acetate buffer at pH 5 for 24 h, followed by the addition of 1 mL of the enzyme cocktail. The total sample volume was completed to 20.0 mL with the same buffer, and the reaction mixture was incubated for different time intervals (2–48 h).
2.5.2 Paper sheet preparation
Initially, the enzymatically treated samples were filtered, washed several times with distilled water, and dried overnight at 70 °C. The treated and the non-treated fibers (blank) were dispersed in water for paper sheets making following our previous work [35].
2.5.3 Assessing of deinking process
Whiteness index of the paper sheets were evaluated using Ultra Scan Pro, (Hunter Lab, USA) spectrophotometer supplied with pulsed xenon lamps as a light source 10O observers with D65 illuminant, d/2 viewing geometry, with measurement area of 2 mm. Ash content was estimated according to TAPPI protocol T 211 om-93, and T 413 om-93 [48]. Other characterization techniques including SEM, FTIR, and EDX analysis were carried out as well.
3 Results and discussion
3.1 Production of enzyme cocktail
Fungi, in general, are well known as common potent producers of various valuable industrially hemicellulytic and cellulytic enzymes with a great scope on the genus Trichoderma and Aspergillus as promising producers for commercial applications. One of the crucial barriers against the industrial applications of these enzymes is the high cost of the commercially available ones [49, 50]. Exploitation of the lignocellulosic biomasses as substrates represents an efficient approach for minimizing the production cost of the targeted enzymes [51,52,53,54,55]. Therefore, the current study focused on the production of cellulytic and hemicellulytic-rich cocktail by applying a local isolate of Aspergillus terreus. As tabulated in Table 1, under the described experimental conditions represented in Sect. 2.2.1, the estimated xylanase activity was 407.959 U/mg protein, while the cellulase (CMCase) activity was 16.466U/ mg protein. The recorded activities in the present study were higher than the previously reported values; 3.5 U/mg CMCase activity was reported for a mutant of Trichoderma afroharzianum [56], and 105.92 U/mg xylanase activity was reported for Aspergillus flavus AW1 [12].
3.2 Hydrolytic activity of the produced cocktail
Herein, the cellulytic and hemicellulytic activity of the produced cocktail was explored by applying the synthetic exo-activity substrates. The recorded results pointed the very low exo-activity in compare to the activity estimated by using CMC and xylan (Table 1), suggesting the endo-activity of the produced enzymes. TLC analysis of the polysaccharide hydrolyzates indicated the production of a mixture of both cello- and xylooligosaccharides released from the enzymatic hydrolysis of CMC and xylan, respectively, sustaining the promising applicability of the present enzymatic cocktail for the production of oligosaccharides (Fig. 2). Nowadays, a growing interest has been oriented for the economic production of oligosaccharides from lignocellulosic polysaccharide constituents as high added-value products capable for improving human and animal health [16].
3.3 Hydrolysis of maize stalks polysaccharide constituents
3.3.1 Hydrolysis of maize stalks biomass
The applicability of the enzymatic cocktail for the hydrolysis of maize stalks after different hydrolysis periods was examined using 10% of the aforementioned cocktail. The results presented in Fig. 3A clearly indicated the enhancement of the hydrolysis yield for the un-treated sample by increasing the incubation time to reach its highest value (7.9%) after 18-h interval. Generally, lignocellulosic biomasses are characterized by their high recalcitrance in their structure which hinders the enzymatic hydrolysis, and consequently, physical or chemical pretreatments have been extensively recommended to reduce this recalcitrance [32, 57] which in turn supposed to improve the yield of the enzymatic hydrolysis of maize stalks. In the current study, microwave pretreatment had been compared to alkaline treatment. The recovery percentage was initially estimated as 53.6 and 66.8% for alkaline, and microwave pretreated biomasses, respectively. Jablonowski et al. [31] reported similar results for the treatment of Agropyron elongatum biomass. Results recorded from the enzymatic hydrolysis of the treated samples indicated that both pretreatments improved the hydrolysis percentage by about 10.4 and 15.2% after 18 h for alkaline and microwave pretreatments, respectively. The improved hydrolysis yield might be attributed to the hydrolysis of the lignin fraction and, consequently, high residual polysaccharide fraction in the remaining biomass, in addition to surface area expansion which improve the accessibility to enzyme-binding sites at the pretreated substrate surface [31, 58]. Moreover, the impact of the added enzyme percentage was examined (Fig. 3B), where the hydrolysis yield improved at higher content of the added enzyme up to 50%, at which the highest hydrolysis percentage (25.1%) was achieved for microwave pretreated samples. It can be concluded that the hydrolysis percentage increased by about 3.2-fold by microwave pretreatment of the sample compared to two-fold increase estimated by Klangpetch et al. [32] for the enzymatic hydrolysis of rice husk. Microwave processing has attracted the research focus as it can facilitate the utilization of various biomasses with a protective manner for its constitutive components [26, 30, 32, 58].
A The impact of the hydrolysis time, B the added amount of the enzymatic cocktail on the hydrolysis of maize stalks in which the 100% represented xylanase activity of 407.959 U/mg protein and cellulase activity of 16.466U/mg protein, and C the image of the TLC plate for the hydrolyzates produced at the optimum conditions from un-treated maize stalks (S), alkaline (A), and microwave-treated (M) samples
TLC analysis (Fig. 3C) of the hydrolyzates resulted from the hydrolysis of the treated and the un-treated maize stalks hydrolyzed for 18 h by the addition of 50% of the enzyme cocktail estimated the release of a mixture of mono- and oligosaccharides. Moreover, HPLC analysis shown in Fig. 4 estimated that the direct hydrolysis of the un-treated maize stalks resulted in the release of monosugar-rich hydrolyzate (glucose, 2.19 mg/mL; xylose, 2.07 mg/mL; and arabinose, 0.25 mg/mL), with the detection of weak bands for cellobiose and xylotriose. The alkaline pretreatment led to the release of xylobiose (1.16 mg/mL)- and xylotriose (0.82 mg/mL)-rich hydrolyzate. On the other hand, microwave pretreatment resulted in releasing of a mixture composed of (cellobiose, 1.82 mg/mL; xylobiose, 0.45 mg/mL; glucose, 1.57 mg /mL; xylose, 1.1 mg/mL; and arabinose, 0.5 mg/mL).
FTIR spectrum shown in Fig. 5 estimated that all of the hydrolyzates possessed typical carbohydrate vibration profile; peaks around 3300 cm−1 were due to OH stretching; near 2900 cm−1 indicated stretching of C–H bonds; peaks between 1200 and 1500 cm−1 based on the bending of C–O, C–H, or C–OH bonds; peaks between 950 and 1200 cm−1 were for C–C, C–O, or C–OH stretching; another peak at 540 cm−1 attributed for C–O–C bending [59]; and finally the peak at about 1600 cm−1 might be due to the bending of the attached water molecules within the hydrolyzate [60].
The total phenolic content in the hydrolyzates was assayed and interpreted on the base of the gallic acid standard curve shown in Fig. 6; the results (Table 2) indicated a total phenolic content in the hydrolyzate of the microwave pretreated sample of 13.118 mg GAE/g dry hydrolyzate which was higher than 10.291 and 12.988 mg GAE/g dry hydrolyzate estimated for the alkaline pretreated and the un-treated samples, respectively. In addition, it was higher than 12.765 mg GAE/g dry weight reported for the alkaline extracted sample of maize husk [61].
The surface morphology of maize stalks was examined (Fig. 7) with a remarkable structural change. The surface of the un-treated sample possessed the packed structure of plants cell wall, and by its enzymatic hydrolysis, slight appearance of cellulosic bundles was estimated. In general, the native form of lignocellulosic biomasses resists enzymatic hydrolysis, and alkaline pretreatment had been previously reported as an efficient tool for the removing of lignin without degrading the carbohydrate content leading to porosity and surface area increase and consequently higher susceptibility to enzymatic hydrolysis [62]. In the alkaline-treated samples, cellulosic fibers were appeared clearly with the observation of the hemicellulosic particles on the surface that disappeared by enzymatic treatment explaining the production of xylooligosaccharide-rich hydrolyzate. In addition, microwave pretreatment had been reported as an efficient process capable for inducing the fragmentation and swelling of the lignocellulosic biomasses and consequently the degradation of the hemicellulose and lignin fractions [63]. Therefore, microwave pretreatment of maize stalk led to the loss of its recalcitrance structure, and after enzymatic treatment, an observed degradation was observed.
Lignocellulosic biomasses are cost-effective and renewable resources for the green production of many valuable products such as sugars and furfurals [14], in addition to phenolic compounds [15] and oligosaccharides [16] that can extensively be exploited in several biotechnological applications.
3.3.2 Hydrolysis of the extracted hemicellulose
Hemicellulose is an economic biocompatible natural polymer with a growing interest for its incorporation in various applications [63]. FTIR analysis of the alkaline extracted hemicellulose fraction of maize stalks estimated that it possessed similar pattern to commercial xylan with an absorption peaks around 3300 cm−1 corresponded to OH stretching, peak at about 2900 cm−1 attributed to C–H stretching. Moreover, peaks around 1600 cm−1 due to bending of the attached water molecules; peak around 1400 cm−1 attributed to C–O, C–OH, or C–H bond bending; peaks around 1030 cm−1 attributed to stretching of C–C, C–O, or C–OH bonds; peak around 900 cm−1 characteristic for the beta glycosidic linkage; peak around 620 cm−1 based on stretching of the C–C–H; and the peak around 500 cm−1 was due to bending of C–O–C (Fig. 8). The moisture content of the air-dried fraction was estimated to be 20%.
In the last few years, the production of xylooligosaccharides by enzymatic hydrolysis of xylan was reported to possess promising biological effects as inhibitors of inflammatory response [23], prebiotic [64], with marked antitumor and antioxidant activities [24]. Thus, enzymatic hydrolysis of the alkaline extracted hemicellulosic fraction was carried out for different hydrolysis periods in compare to commercial xylan. As illustrated (Fig. 9A), the optimal hydrolysis period was 4 h with hydrolysis percentage of 71% estimated for maize stalks hemicellulose in compare to 38% estimated for commercial xylan. Moreover, the hydrolysis of maize stalks hemicellulose fraction using different concentrations of the enzyme cocktail was examined with a sharp improvement in the hydrolysis yield up to enzyme concentration 5%, with a slight increase afterward (Fig. 9B). The water solubility of the extracted fraction was 10%, and by its enzymatic hydrolysis, the hydrolyzate was completely water soluble. In addition, TLC plate for the produced hydrolyzate produced was carried out in compare to the hydrolysis of commercial xylan under the same optimal conditions (using 5% enzyme concentration for 4 h). The results (Fig. 9C) estimated the release of a mixture of mono and oligosaccharides in which xylobiose was the main product as confirmed by HPLC (Fig. 10). Xylobiose content in the hydrolyzate of the hemicellulose fraction was 1.94 mg/mL that was 63% higher than 1.19 mg/mL estimated in commercial xylan hydrolyzate. FTIR analysis of the produced hydrolyzates possessed similar pattern to the parent compounds with an observed increase in the intensity of the peaks. Moreover, the total phenolic content in the hydrolyzate of the hemicellulose fraction and that of xylan was 9.399 and 2.766 mg GAE/g dry hydrolyzate, respectively.
3.3.3 Antioxidant activity of the hydrolyzate
The scavenging activity of the produced hydrolyzate against DPPH radical was evaluated (Table 3). All the tested maize stalk hydrolyzates possessed antioxidant activity with a quite variation in which the hydrolyzates produced upon the hydrolysis of microwave pretreated maize stalks possessed the highest antioxidant activity. In general, such antioxidant activity of various lignocellulosic biomasses hydrolyzates can be attributed to the presence of either oligosaccharides or phenolic compounds [23, 65, 66]. Herein, the hydrolyzate that possessed the highest oligosaccharide and phenolic contents exhibited the highest antioxidant activity. Additionally, the hydrolyzate that produced from the hydrolysis of the extracted hemicellulose fraction possessed 3.4 higher activity than the hydrolyzate obtained from commercial xylan. Zheng et al. [67] estimated that the phenolic compounds present in the structure of xylan exerted an antioxidant activity.
3.4 Bio-deinking of office waste paper
3.4.1 Optimization of the pretreatment conditions
The potential applicability of the produced cocktail for the bio-deinking of office waste paper was evaluated. Preliminary, the optimum deinking period, as well as the amount of the enzyme cocktail added, was monitored via following of the released reducing sugars in the supernatant. As presented in Tables 4 and 5, the highest amount of the released reducing sugars was recorded after 24 h using 1.5 mL of the enzyme cocktail without the detection of any amount of reducing sugars in the supernatant of the buffer-treated sample for 24 h without the addition of the enzymatic cocktail. The sample produced at the optimized conditions was used and compared with the buffer-treated and the blank paper sheet in the deinking evaluation.
3.4.2 Assessing of deinking process
Toners are powdered mixtures used to transfer printed text and images to paper in laser printers and copiers, usually in toner cartridges. They are usually consisted of suitable thermoplastic adhesives which polymerized and fused on the paper fibers during the temperature-based printing process and the early mixtures composed mainly of carbon and iron oxide powder [68]. After printing, the precipitated toners on the paper surface remain as bulky, stiff, and flat components which cannot be easily removed by applying the usual flotation techniques during the traditional chemical deinking process [35]. Therefore, the enzymatic deinking process had attracted the research focus [33]. The evaluation of the enzymatic deinking process was carried out considering the whiteness index, ash content, SEM, and EDX analysis for the 24 h treated sample in comparison to the buffer-treated sample and a blank one. Results presented in Fig. 11A clearly indicated the improvement of the whiteness index percentage by 34% for the enzyme-treated sample, whereas the buffer-treated sample shown a small increase in the whiteness index by about 6%. In addition, the ash content was determined, and a significant decrease of about 80% was estimated in comparison with the blank sheet. However, for the buffer-treated sample, the ash content was not significantly changed. Moreover, the FTIR spectra shown in Fig. 11B admitted the observation of slight changes in the enzyme-treated sample including the disappearance of band at 2850 cm−1 in addition to noticeable enhancement of the band at 872 cm−1. Herein, the SEM and EDX affirmed the above conclusion as shown in Fig. 12. The SEM image clarified that the fibers of the blank paper sheet and that of the buffer-treated one were loaded with ink particles that obviously decreased after enzymatic treatment. The EDX chart recorded the elemental composition of the blank paper sheet as carbon, oxygen, calcium, and iron. The paper sheet of the enzymatically treated sample processed for 24 h using 1.5 mL of the produced cocktail possessed the same elemental composition of the blank one with a reduction in the iron percentage in compare to a very low value of change (0.17%) estimated for the sample treated with buffer only. These findings emphasized the efficiency of the examined enzyme cocktail in the deinking of office waste paper.
4 Conclusion
The current study focused on the enzymatic valorization of cellulosic and hemicellulosic-based biomasses. Initially, the preparation of water-soluble antioxidant hydrolyzate of maize stalks was carried out using different strategies. The hydrolyzates resulted from the microwave pretreated samples were rich in oligosaccharides (cellobiose and xylobiose); in addition, it possessed the highest phenolic content and consequently exhibited the highest antioxidant activity. Moreover, the hydrolyzate produced from the enzymatic hydrolysis of the alkaline extracted hemicellulosic fraction was rich in xylooligosaccharides and phenolic content higher than that estimated for the hydrolysis of commercial xylan with higher antioxidant activity. Furthermore, the potential applicability of the produced enzymatic cocktail for the eco-friendly deinking of office waste paper sheets was evaluated with the observation of efficient dislodgement ability for the printed ink from the paper surface affirmed on the base of the physiochemical and topographical analysis.
Data availability
All the data available in the following manuscript are fundamental.
References
Tatta ER, Imchen M, Moopantakath J, Kumavath R (2022) Bioprospecting of microbial enzymes: current trends in industry and healthcare. Appl Microbiol Biotechnol 106(5–6):1813–1835
Bhardwaj N, Kumar B, Agrawal K, Verma P (2021) Current perspective on production and applications of microbial cellulases: a review. Bioresour Bioproc 8:1–34
Allam O, Ismail S, Abdelghaffar R, Mashaly H, Hassan A (2020) The impact of egyptian thermophylic cellulase on the dyeability of natural and recovered cellulosic fabrics. Egypt J Chem 63(9):3391–3400
Mohi MA, Ismail S (2022) Assessment of the applicability of cellulolytic enzyme in disassembling of caked papers. Egypt J Chem 65(1):581–591
Mohamed WSA, Mohie MA, Ismail SA, Hassan AA (2022) Dismantling the linen clumping paper by using cellulytic enzyme. Bio Res Appl Chem 13:1–15
Ebringerová A, Heinze T (2000) Xylan and xylan derivatives–biopolymers with valuable properties, Naturallyoccurring xylans structures, isolation procedures and properties. Macromol Rapid Comm 21(9):542–556
da Silva PO, de AlencarGuimaraes NC, Serpa JDM, Masui DC, Marchetti CR, Verbisck NV, Giannesi GC (2019) Application of an endo-xylanase from Aspergillus japonicus in the fruit juice clarification and fruit peel waste hydrolysis. Biocatal Agric Biotechnol 21:101312
Althuri A, Mohan SV (2019) Single pot bioprocessing for ethanol production from biogenic municipal solid waste. Bioresour Technol 283:159–167
Dhivahar J, Khusro A, Paray BA, Rehman MU, Agastian P (2020) Production and partial purification of extracellular xylanase from Pseudomonas nitroreducens using frugivorous bat (Pteropus giganteus) faeces as ideal substrate and its role in poultry feed digestion. J King Saud Univ Sci 32(4):2474–2479
Mhiri S, Bouanane-Darenfed A, Jemli S, Neifar S, Ameri R, Mezghani, et al (2020) A thermophilic and thermostable xylanase from Caldicoprobacteralgeriensis: recombinant expression, characterization and application in paper biobleaching. Int J Biol Macromol 164:808–817
Anand G, Leibman-Markus M, Elkabetz D, Bar M (2021) Method for the production and purification of plant immuno-active xylanase from trichoderma. Int J Molr Sci 22(8):4214
Ismail SA, Nour SA, Hassan AA (2022) Valorization of corn cobs for xylanase production by Aspergillus flavus AW1 and its application in the production of antioxidant oligosaccharides and removal of food stain. Biocatal Agric Biotechnol 41:102311
Zabed H, Sahu JN, Boyce AN, Faruq G (2016) Fuel ethanol production from lignocellulosic biomass: an overview on feedstocks and technological approaches. Renew Sustain Energy Rev 66:751–774
Świątek K, Gaag S, Klier A, Kruse A, Sauer J, Steinbach D (2020) Acid hydrolysis of lignocellulosic biomass: sugars and furfurals formation. Catalysts 10(4):437
Liu C, Cai T, Yin X, Liang J, Jia S, Zhang X, Xu J, Hu J, Jiang J, Wang K (2022) A sustainable and profitable biorefinery strategy for efficiently converting lignocellulose to furfural, glucose and phenolic compounds. Green Chem 24(21):8494–8502
Saini R, Patel AK, Saini JK, Chen CW, Varjani S, Singhania RR, Di Dong C (2022) Recent advancements in prebiotic oligomers synthesis via enzymatic hydrolysis of lignocellulosic biomass. Bioengineered 13(2):2139–2172
Hasanin MS (2022) Cellulose-based biomaterials: chemistry and biomedical applications. Starch-Stärke 74(7–8):2200060
Ávila PF, Silva MF, Martins M, Goldbeck R (2021) Cello-oligosaccharides production from lignocellulosic biomass and their emerging prebiotic applications. World J Microbiol Biotechnol 37:1–11
de Freitas C, Terrone CC, Forsan CF, Milagres AM, Brienzo M (2022) Oligosaccharides from lignocellulosic biomass and their biological and physicochemical properties. In: Brienzo M (ed) Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy. Clean Energy Production Technologies. Springer, Singapore, pp 275–309. https://doi.org/10.1007/978-981-16-3682-0_9
Ismail SA, Hassan AA, Nour SA, El-Sayed HS (2023) The production of stirred yogurt fortified with prebiotic xylooligosaccharide, probiotic and synbiotic microcapsules. Biocatal Agric Biotechnol 13:102729
Bouiche C, Boucherba N, Benallaoua S, Martinez J, Diaz P, Pastor FJ, Valenzuela SV (2020) Differential antioxidant activity of glucuronoxylooligosaccharides (UXOS) and arabinoxylooligosaccharides (AXOS) produced by two novel xylanases. Int J Biol Macromol 155:1075–1083
Ismail SA, Hassan AA, Emran MA (2019) Economic production of thermo-active endo β-mannanase for the removal of food stain and production of antioxidant manno-oligosaccharides. Biocatal Agric Biotechnol 22:101387
Wang Y, Yang Y, Qu Y, Zhang J (2021) Selective removal of lignin with sodium chlorite to improve the quality and antioxidant activity of xylo-oligosaccharides from lignocellulosic biomass. Bioresour Technol 337:125506
Batsalova T, Georgiev Y, Moten D, Teneva I, Dzhambazov B (2022) Natural xylooligosaccharides exert antitumor activity via modulation of cellular antioxidant state and TLR4. Int J Mol Sci 23(18):10430
Aliakbarlu J, Mohammadi S, Khalili S (2014) A study on antioxidant potency and antibacterial activity of water extracts of some spices widely consumed in Iranian diet. J Food Biochem 38(2):159–166
Ismail SA (2019) Microbial valorization of shrimp byproducts via the production of thermostable chitosanase and antioxidant chitooligosaccharides. Biocatal Agric Biotechnol 20:101269
Hasanin MS, Abd El-Aziz ME, El-Nagar I, Hassan YR, Youssef AM (2022) Green enhancement of wood plastic composite based on agriculture wastes compatibility via fungal enzymes. Sci Rep 12(1):19197
Mankar AR, Pandey A, Modak A, Pant KK (2021) Pretreatment of lignocellulosic biomass: a review on recent advances. Bioresour Technol 334:125235
Rodriguez-Jasso RM, Mussatto SI, Pastrana L, Aguilar CN, Teixeira JA (2011) Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr Polym 86(3):1137–1144
Fia AZ, Amorim J (2023) Microwave pretreatment of biomass for conversion of lignocellulosic materials into renewable biofuels. J Energy Inst 106:101146
Jablonowski ND, Pauly M, Dama M (2022) Microwave assisted pretreatment of Szarvasi (Agropyron elongatum) biomass to enhance enzymatic saccharification and direct glucose production. Front Plant Sci 12:3177
Klangpetch W, Pattarapisitporn A, Phongthai S, Utama-Ang N, Laokuldilok T, Tangjaidee P, Wirjantoro TI, Jaichakan P (2022) Microwave-assisted enzymatic hydrolysis to produce xylooligosaccharides from rice husk alkali-soluble arabinoxylan. Sci Rep 12(1):11
Mondal S, Biswal D, Pal K, Rakshit S, Halder SK, Mandavgane SA, Bera D, Hossain M, Mondal KC (2022) Biodeinking of waste papers using combinatorial fungal enzymes and subsequent production of butanol from effluent. Bioresour Technol 353:127078
Biswas P, Bharti AK, Kadam A, Dutt D (2019) Bio-deinking of mixed office waste paper by Penicillium citrinum NCIM-1398 and its comparative study with conventional chemical deinking. BioResources 14(3):6544–6557
Hasanin MS, Hashem AH, Abd El-Sayed ES, El-Saied H (2020) Green ecofriendly bio-deinking of mixed office waste paper using various enzymes from Rhizopus microsporus AH3: efficiency and characteristics. Cellulose 27:4443–4453
Kumar A, Dutt D (2021) A comparative study of conventional chemical deinking and environment-friendly bio-deinking of mixed office wastepaper. Sci Afr 12:00793
Ismail SA, Hassan AA (2020) Optimizing the production of rice straw hydrolytic cellulase under solid-state fermentation using Aspergillus terreus RS2. Egypt Pharm J 19(1):7
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Boil Chem 193:265–275
Rustiguel CB, Jorge JA, Guimarães LHS (2012) Optimization of the chitinase production by different Metarhizium anisopliae strains under solid-state fermentation with silkworm chrysalis as substrate using CCRD. Adv Microbiol 2(03):268
Tanaka T, Fujiwara S, Nishikori S, Fukui T, Takagi M, Imanaka TA (1999) Unique chitinase with dual active sites and triple substrate binding sites from the hyperthermophilic Archaeon Pyrococcus kodakaraenis KODI. Appl Environ Microbiol 15:5338–5344
Zafar A, Hamid A, Peng L, Wang Y, Aftab MN (2022) Enzymatic hydrolysis of lignocellulosic biomass using a novel, thermotolerant recombinant xylosidase enzyme from Clostridium clariflavum: a potential addition for biofuel industry. RSC Adv 12(23):14917–14931
Rose DJ, Inglett GE (2010) Production of feruloylated arabinoxylo-oligosaccharides from maize (Zea mays) bran by microwave-assisted autohydrolysis. Food Chem 119(4):1613–1618
Chen WP, Anderson AW (1980) Extraction of hemicellulose from ryegrass straw for the production of glucose isomerase and use of the resulting straw residue for animal feed. Biotechnol Bioeng 22(3):519–531
Cano-Chauca M, Stringheta PC, Ramos AM, Cal-Vidal J (2005) Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. IFSET 6(4):420–428
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2(4):875–877
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30
Delozier G, Zhao Y, Deng Y, White D, Zhu J, Prein M (2005) Laboratory-and mill-scale study of surfactant spray flotation deinking. Tappi J 4(10):25–30
Shuddhodana XX, Munishwar NG, Virendra SB (2018) Stable cellulolytic enzymes and their application in hydrolysis of lignocellulosic biomass. Biotechnol J 13:1700633
Bhardwaj N, Kumar B, Verma P (2019) A detailed overview of xylanases: an emerging biomolecule for current and future prospective. BIOB 6(1):1–36
Barbieri GS, Bento HB, de Oliveira F, Picheli FP, Dias LM, Masarin F, Santos-Ebinuma VC (2022) Xylanase production by Talaromyces amestolkiae valuing agroindustrial byproducts. Biotechnology 11(2):15
Ezeilo UR, Lee CT, Huyop F, Zakaria II, Wahab RA (2019) Raw oil palm frond leaves as cost-effective substrate for cellulase and xylanase productions by Trichoderma asperellum UC1 under solid-state fermentation. J Environ Manag 243:206–217
Jovanović M, Vučurović D, Bajić B, Dodić S, Vlajkov V, Jevtić-Mučibabić R (2020) Optimization of the simultaneous production of cellulase and xylanase by submerged and solid-state fermentation of wheat chaff. J Serbian Chem Soc 85(2):177–189
Srivastava N, Mohammad A, Pal DB, Srivastava M, Alshahrani MY, Ahmad I, Singh R, Mishra PK, Yoon T and Gupta VK (2022) Enhancement of fungal cellulase production using pretreated orange peel waste and its application in improved bioconversion of rice husk under the influence of nickel cobaltite nanoparticles. Biomass Convers Biorefin 1–10. https://doi.org/10.1007/s13399-022-03070-3
Tai WY, Tan JS, Lim V, Lee CK (2019) Comprehensive studies on optimization of cellulase and xylanase production by a local indigenous fungus strain via solid state fermentation using oil palm frond as substrate. Biotechnol Prog 35(3):2781
Peng ZQ, Li C, Lin Y, Wu SS, Gan LH, Liu J, Yang SL, Zeng XH, Lin L (2021) Cellulase production and efficient saccharification of biomass by a new mutant Trichoderma afroharzianum MEA-12. Biotechnol Biofuels 14(1):1–13
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48
Hoang AT, Nižetić S, Ong HC, Mofijur M, Ahmed SF, Ashok B, Chau MQ (2021) Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 281:130878
Yang K, Zhang Y, Cai M, Guan R, Neng J, Pi X, Sun P (2020) In vitro prebiotic activities of oligosaccharides from the by-products in Ganoderma lucidum spore polysaccharide extraction. RSC Adv 10(25):14794–14802
Jnawali P, Kumar V, Tanwar B, Hirdyani H, Gupta P (2018) Enzymatic production of xylooligosaccharides from brown coconut husk treated with sodium hydroxide. Waste Biomass Valoriz 9(10):1757–1766
Vazquez-Olivo G, López-Martínez LX, Contreras-Angulo L, Heredia JB (2019) Antioxidant capacity of lignin and phenolic compounds from corn stover. Waste Biomass Valoriz 10:95–102
Puligundla P, Oh SE, Mok C (2016) Microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to sugars and ethanol: a review. Carbon Lett 17(1):1–10
Xu Y, Liu K, Yang Y, Kim MS, Lee CH, Zhang R, Xu T, Choi SE, Si C (2022) Hemicellulose-based hydrogels for advanced applications. Front Bioeng Biotechnol 10:1110004
Yan B, Huang C, Lai C, Ling Z, Yong Q (2022) Production of prebiotic xylooligosaccharides from industrialderived xylan residue by organic acid treatment. Carbohydr Polym 292:119641
Si D, Shang T, Liu X, Zheng Z, Hu Q, Hu C, Zhang R (2020) Production and characterization of functional wheat bran hydrolysate rich in reducing sugars, xylooligosaccharides and phenolic acids. Biotechnol Rep 27:e00511
Silva VTF, Ruschoni UC, Ferraz A and Milagres AM (2022) Xylan, xylooligosaccharides, and aromatic structures with antioxidant activity released by xylanase treatment of alkaline-sulfite–pretreated sugarcane bagasse. Front Bioeng Biotechnol 10:940712
Zheng L, Yu P, Zhang Y, Wang P, Yan W, Guo B, Huang C, Jiang Q (2021) Evaluating the bio-application of biomacromolecule of lignin-carbohydrate complexes (LCC) from wheat straw in bone metabolism via ROS scavenging. Int J Biol Macromol 176:13–25
Nikolaev A (2019) Flotation recovery of toner containing iron oxide from water suspension. Miner Eng 144:106027
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design as well as methodology. The first draft of the manuscript was written by Shaymaa A. Ismail, and all authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, A.A., Hasanin, M.S. & Ismail, S.A. Enzymatic valorization of cellulosic and hemicellulosic-based biomasses via the production of antioxidant water-soluble hydrolyzate of maize stalks and the green bio-deinking of mixed office waste paper. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04798-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04798-2