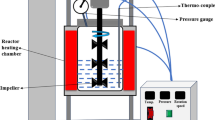
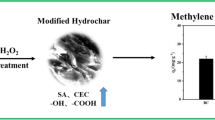
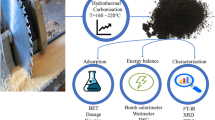
Abstract
This study focuses on the valorization of biomass waste from the fragrance industry in Provence, France. Adsorbents enriched with oxygen surface groups were produced from Lavandin (Lavandula genus) using the hydrothermal carbonization (HTC) process followed by further NaOH surface treatment. The original Lavandin hydrochar (LH) and NaOH surface treated Lavandin hydrochar (NaOH-LH) were systematically characterized by SEM, FTIR, elemental analysis, zeta potential, and Boehm titration. Specifically, the NaOH surface treatment scavenged the heterogeneous fragments adhering to the surface, increased the number of oxygen surface groups and optimized the ratio of these groups without damaging the hydrochar skeleton structure. Batch adsorption tests for the representative contaminant methylene blue (MB) showed that the adsorption process can be well described by the pseudo-second-order kinetic model and the Langmuir thermodynamic model. In neutral aqueous solution, the three tested adsorbents LH, 0.1%NaOH-LH, and 4%NaOH-LH had adsorption capacities of 52 mg·g−1, 137 mg·g−1, and 306 mg·g−1 for MB. Adsorption experiments allowed us to elucidate that the adsorption mechanism was dominated by electrostatic attractions, supplemented by hydrogen bonds, π-π interactions, and n-π interactions. At some conditions, these capacities overcame the surface density of available adsorption sites, which was attributed to the steric hindrance between stacked adsorbed MB molecules, as the main mechanism governing the maximal surface density of the adsorbed monolayer. This study proves that the combination of the HTC process with NaOH surface treatment method converts Lavandin biomass waste into an efficient adsorbent for MB, providing an economic and ecological alternative for its valorization.








Similar content being viewed by others
Data availability
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Lesage-Meessen L, Bou M, Sigoillot JC et al (2015) Essential oils and distilled straws of lavender and lavandin: a review of current use and potential application in white biotechnology. Appl Microbiol Biotechnol 99:3375–3385. https://doi.org/10.1007/s00253-015-6511-7
Périno-Issartier S, Ginies C, Cravotto G, Chemat F (2013) A comparison of essential oils obtained from lavandin via different extraction processes: ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. J Chromatogr A 1305:41–47. https://doi.org/10.1016/J.CHROMA.2013.07.024
Torras-Claveria L, Jauregui O, Bastida J et al (2007) Antioxidant activity and phenolic composition of lavandin (Lavandula x intermedia Emeric ex Loiseleur) waste. J Agric Food Chem 55:8436–8443. https://doi.org/10.1021/jf070236n
Tiliacos C, Gaydou EM, Bessière J-M, Agnel R (2008) Distilled lavandin (Lavandula intermedia Emeric ex Loisel) wastes: a rich source of coumarin and herniarin. J Essent Oil Res 20:412–413. https://doi.org/10.1080/10412905.2008.9700043
Cho EJ, Trinh LTP, Song Y et al (2020) Bioconversion of biomass waste into high value chemicals. Bioresour Technol 298:122386. https://doi.org/10.1016/J.BIORTECH.2019.122386
Nguyen TN, Le PA, Phung VBT (2022) Facile green synthesis of carbon quantum dots and biomass-derived activated carbon from banana peels: synthesis and investigation. Biomass Convers Biorefin 12:2407–2416. https://doi.org/10.1007/s13399-020-00839-2
Bakar NA, Othman N, Yunus ZM et al (2021) Nipah (Musa Acuminata Balbisiana) banana peel as a lignocellulosic precursor for activated carbon: characterization study after carbonization process with phosphoric acid impregnated activated carbon. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01937-5
Sharma HB, Sarmah AK, Dubey B (2020) Hydrothermal carbonization of renewable waste biomass for solid biofuel production: a discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew Sustain Energy Rev 123:109761. https://doi.org/10.1016/J.RSER.2020.109761
Suriapparao D, Tejasvi R (2022) A review on role of process parameters on pyrolysis of biomass and plastics: present scope and future opportunities in conventional and microwave-assisted pyrolysis technologies. Process Saf Environ Prot 162:435–462. https://doi.org/10.1016/J.PSEP.2022.04.024
Wang T, Zhai Y, Zhu Y et al (2018) A review of the hydrothermal carbonization of biomass waste for hydrochar formation: process conditions, fundamentals, and physicochemical properties. RenewSustain Energy Rev 90:223–247. https://doi.org/10.1016/J.RSER.2018.03.071
Zhuang X, Liu J, Zhang Q et al (2022) A review on the utilization of industrial biowaste via hydrothermal carbonization. RenewSustain Energy Rev 154:111877. https://doi.org/10.1016/J.RSER.2021.111877
Heidari M, Dutta A, Acharya B, Mahmud S (2019) A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J Energy Inst 92:1779–1799. https://doi.org/10.1016/J.JOEI.2018.12.003
Zhang Z, Yang J, Qian J et al (2021) Biowaste hydrothermal carbonization for hydrochar valorization: skeleton structure, conversion pathways and clean biofuel applications. Bioresour Technol 324:124686. https://doi.org/10.1016/J.BIORTECH.2021.124686
Li X, Baran SS, Orange F et al (2022) Conversion of lavandula straw into high-quality solid fuel: effect of hydrothermal carbonization conditions on fuel characteristics. Bioenergy Res. https://doi.org/10.1007/s12155-022-10492-4
Hwang H, Lee JH, Ahmed MA, Choi JW (2021) Evaluation of pyrochar and hydrochar derived activated carbons for biosorbent and supercapacitor materials. J Environ Manage 298:113436. https://doi.org/10.1016/J.JENVMAN.2021.113436
Mariuzza D, Lin JC, Volpe M et al (2022) Impact of Co-Hydrothermal carbonization of animal and agricultural waste on hydrochars’ soil amendment and solid fuel properties. Biomass Bioenergy 157:106329. https://doi.org/10.1016/J.BIOMBIOE.2021.106329
Shi Y, Zhang T, Ren H et al (2018) Polyethylene imine modified hydrochar adsorption for chromium (VI) and nickel (II) removal from aqueous solution. Bioresour Technol 247:370–379. https://doi.org/10.1016/J.BIORTECH.2017.09.107
Nkuigue Fotsing P, Bouazizi N, Djoufac Woumfo E et al (2021) Investigation of chromate and nitrate removal by adsorption at the surface of an amine-modified cocoa shell adsorbent. J Environ Chem Eng 9:104618. https://doi.org/10.1016/J.JECE.2020.104618
Jais FM, Ibrahim S, Chee CY, Ismail Z (2021) High removal of crystal violet dye and tetracycline by hydrochloric acid assisted hydrothermal carbonization of sugarcane bagasse prepared at high yield. Sustain Chem Pharm 24:100541. https://doi.org/10.1016/J.SCP.2021.100541
Qu J, Lin X, Liu Z et al (2022) One-pot synthesis of Ca-based magnetic hydrochar derived from consecutive hydrothermal and pyrolysis processing of bamboo for high-performance scavenging of Pb(II) and tetracycline from water. Bioresour Technol 343:126046. https://doi.org/10.1016/J.BIORTECH.2021.126046
Li SY, Teng HJ, Guo JZ et al (2021) Enhanced removal of Cr(VI) by nitrogen-doped hydrochar prepared from bamboo and ammonium chloride. Bioresour Technol 342:126028. https://doi.org/10.1016/J.BIORTECH.2021.126028
Wei Y, Fakudze S, Zhang Y et al (2022) Co-hydrothermal carbonization of pomelo peel and PVC for production of hydrochar pellets with enhanced fuel properties and dechlorination. Energy 239:122350. https://doi.org/10.1016/J.ENERGY.2021.122350
Khoshbouy R, Takahashi F, Yoshikawa K (2019) Preparation of high surface area sludge-based activated hydrochar via hydrothermal carbonization and application in the removal of basic dye. Environ Res 175:457–467. https://doi.org/10.1016/J.ENVRES.2019.04.002
Tu W, Liu Y, Xie Z et al (2021) A novel activation-hydrochar via hydrothermal carbonization and KOH activation of sewage sludge and coconut shell for biomass wastes: Preparation, characterization and adsorption properties. J Colloid Interface Sci 593:390–407. https://doi.org/10.1016/J.JCIS.2021.02.133
Mendoza Martinez CL, Sermyagina E, Saari J et al (2021) Hydrothermal carbonization of lignocellulosic agro-forest based biomass residues. Biomass Bioenergy 147:106004. https://doi.org/10.1016/J.BIOMBIOE.2021.106004
Liu L, Sim SF, Lin S et al (2021) Integrated structural and chemical analyses for HCl-supported hydrochar and their adsorption mechanisms for aqueous sulfachloropyridazine removal. J Hazard Mater 417:126009. https://doi.org/10.1016/J.JHAZMAT.2021.126009
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod Biorefin 4:160–177. https://doi.org/10.1002/bbb.198
Sun K, Tang J, Gong Y, Zhang H (2015) Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ Sci Pollut Res 22:16640–16651. https://doi.org/10.1007/s11356-015-4849-0
Oladoye PO, TO A, Omotola EO, Oyewola OJ (2022) Methylene blue dye: toxicity and potential elimination technology from wastewater. Results in Eng 16:100678. https://doi.org/10.1016/J.RINENG.2022.100678
Jawad AH, Abdulhameed AS, Hanafiah MAKM et al (2021) Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean J Chem Eng 38:1499–1509. https://doi.org/10.1007/s11814-021-0801-9
Jawad AH, Saud Abdulhameed A, Wilson LD et al (2021) High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: optimization and mechanism study. Chin J Chem Eng 32:281–290. https://doi.org/10.1016/J.CJCHE.2020.09.070
Laranja MJ, da Silva RCJ, Bisinoti MC et al (2022) Factorial design of experiments for extraction and screening analysis of organic compounds in hydrochar and its process water of sugar cane bagasse and vinasse. Biomass Convers Biorefin 12:81–90. https://doi.org/10.1007/s13399-020-01035-y
Boehm H-P, Diehl E, Heck W, Sappok R (1964) Surface oxides of carbon. Angew Chem Int Ed Engl 3:669–677. https://doi.org/10.1002/anie.196406691
Boehm HP (1994) Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon N Y 32:759–769. https://doi.org/10.1016/0008-6223(94)90031-0
Boehm HP (2002) Surface oxides on carbon and their analysis: a critical assessment. Carbon N Y 40:145–149. https://doi.org/10.1016/S0008-6223(01)00165-8
Schönherr J, Buchheim J, Scholz P, Adelhelm P (2018) Boehm titration revisited (part I): practical aspects for achieving a high precision in quantifying oxygen-containing surface groups on carbon materials. C 4:21. https://doi.org/10.3390/c4020021
Wang R, Jin Q, Ye X et al (2020) Effect of process wastewater recycling on the chemical evolution and formation mechanism of hydrochar from herbaceous biomass during hydrothermal carbonization. J Clean Prod 277:123281. https://doi.org/10.1016/J.JCLEPRO.2020.123281
Zhang Y, Jiang Q, Xie W et al (2019) Effects of temperature, time and acidity of hydrothermal carbonization on the hydrochar properties and nitrogen recovery from corn stover. Biomass Bioenergy 122:175–182. https://doi.org/10.1016/J.BIOMBIOE.2019.01.035
Śliz M, Wilk M (2020) A comprehensive investigation of hydrothermal carbonization: energy potential of hydrochar derived from Virginia mallow. Renew Energy 156:942–950. https://doi.org/10.1016/J.RENENE.2020.04.124
Gascó G, Paz-Ferreiro J, Álvarez ML et al (2018) Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag 79:395–403. https://doi.org/10.1016/J.WASMAN.2018.08.015
Liu Y, Ma S, Chen J (2018) A novel pyro-hydrochar via sequential carbonization of biomass waste: Preparation, characterization and adsorption capacity. J Clean Prod 176:187–195. https://doi.org/10.1016/J.JCLEPRO.2017.12.090
Gao Y, Wang X, Wang J et al (2013) Effect of residence time on chemical and structural properties of hydrochar obtained by hydrothermal carbonization of water hyacinth. Energy 58:376–383. https://doi.org/10.1016/J.ENERGY.2013.06.023
Ghanim BM, Pandey DS, Kwapinski W, Leahy JJ (2016) Hydrothermal carbonisation of poultry litter: Effects of treatment temperature and residence time on yields and chemical properties of hydrochars. Bioresour Technol 216:373–380. https://doi.org/10.1016/J.BIORTECH.2016.05.087
Fan J, Li F, Fang D et al (2022) Effects of hydrophobic coating on properties of hydrochar produced at different temperatures: Specific surface area and oxygen-containing functional groups. Bioresour Technol 363:127971. https://doi.org/10.1016/J.BIORTECH.2022.127971
Kitamura H, Sekido M, Takeuchi H, Ohno M (2011) The method for surface functionalization of single-walled carbon nanotubes with fuming nitric acid. Carbon N Y 49:3851–3856. https://doi.org/10.1016/J.CARBON.2011.05.020
Tang J, Mu B, Zong L et al (2017) Facile and green fabrication of magnetically recyclable carboxyl-functionalized attapulgite/carbon nanocomposites derived from spent bleaching earth for wastewater treatment. J Chem Eng 322:102–114. https://doi.org/10.1016/J.CEJ.2017.03.116
Wang P, Tang Y, Liu Y et al (2018) Halloysite nanotube@carbon with rich carboxyl groups as a multifunctional adsorbent for the efficient removal of cationic Pb(ii), anionic Cr(vi) and methylene blue (MB). Environ Sci Nano 5:2257–2268. https://doi.org/10.1039/c8en00561c
Ho YS, Wase D, Forster CF (1996) Removal of lead ions from aqueous solution using sphagnum moss peat as adsorbent. Water SA 22:219–224. https://doi.org/10.1039/JR9600003973
Giles H, Macewan TH, Nakhwa SN, Smith D (1960) A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc 111:3973–3993. https://doi.org/10.1039/JR9600003973
Wang J, Guo X (2020) Adsorption isotherm models: classification, physical meaning, application and solving method. Chemosphere 258:127279. https://doi.org/10.1016/J.CHEMOSPHERE.2020.127279
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: A review. J Hazard Mater 393:122383. https://doi.org/10.1016/J.JHAZMAT.2020.122383
Ahmed MB, Zhou JL, Ngo HH et al (2018) Sorption of hydrophobic organic contaminants on functionalized biochar: Protagonist role of π-π electron-donor-acceptor interactions and hydrogen bonds. J Hazard Mater 360:270–278. https://doi.org/10.1016/J.JHAZMAT.2018.08.005
Dotto GL, Santos JMN, Rodrigues IL et al (2015) Adsorption of Methylene Blue by ultrasonic surface modified chitin. J Colloid Interface Sci 446:133–140. https://doi.org/10.1016/J.JCIS.2015.01.046
Prasannamedha G, Kumar PS, Mehala R et al (2021) Enhanced adsorptive removal of sulfamethoxazole from water using biochar derived from hydrothermal carbonization of sugarcane bagasse. J Hazard Mater 407:124825. https://doi.org/10.1016/J.JHAZMAT.2020.124825
Talbot D, Queiros Campos J, Checa-Fernandez BL et al (2021) Adsorption of organic dyes on magnetic iron oxide nanoparticles. Part I: mechanisms and adsorption-induced nanoparticle agglomeration. ACS Omega 6:19086–19098. https://doi.org/10.1021/acsomega.1c02401
Bollinger JC (2020) Letter to the Editor: Comments on “Adsorption of methylene blue and Cd(II) onto maleylated modified hydrochar from water.”. Environ Pollut 261:113824. https://doi.org/10.1016/J.ENVPOL.2019.113824
Tran HN, You SJ, Nguyen TV, Chao HP (2017) Insight into the adsorption mechanism of cationic dye onto biosorbents derived from agricultural wastes. Chem Eng Commun 204:1020–1036. https://doi.org/10.1080/00986445.2017.1336090
Bushra R, Mohamad S, Alias Y et al (2021) Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: a review. Microporous and Mesoporous Materials 319:111040. https://doi.org/10.1016/J.MICROMESO.2021.111040
Hang PT, Brindley GW (1970) Methylene blue absorption by clay minerals. determination of surface areas and cation exchange capacities (clay-organic studies xvid). Clays Clay Miner 18:203–212. https://doi.org/10.1346/CCMN.1970.0180404
Seung Kim Y, Rae Park C (2016) Titration method for the identification of surface functional groups. In: Materials science and engineering of carbon: characterization. Elsevier, pp 273–286
Ovchinnikov O, Evtukhova AV, Kondratenko TS et al (2016) Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib Spectrosc 86:181–189. https://doi.org/10.1016/J.VIBSPEC.2016.06.016
Yan H, Tao X, Yang Z et al (2014) Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J Hazard Mater 268:191–198. https://doi.org/10.1016/J.JHAZMAT.2014.01.015
Ronix A, Pezoti O, Souza LS et al (2017) Hydrothermal carbonization of coffee husk: optimization of experimental parameters and adsorption of methylene blue dye. J Environ Chem Eng 5:4841–4849. https://doi.org/10.1016/J.JECE.2017.08.035
Islam MA, Ahmed MJ, Khanday WA et al (2017) Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol Environ Saf 138:279–285. https://doi.org/10.1016/J.ECOENV.2017.01.010
Madduri S, Elsayed I, Hassan EB (2020) Novel oxone treated hydrochar for the removal of Pb(II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 260:127683. https://doi.org/10.1016/J.CHEMOSPHERE.2020.127683
Liu JL, Qian WC, Guo JZ et al (2021) Selective removal of anionic and cationic dyes by magnetic Fe3O4-loaded amine-modified hydrochar. Bioresour Technol 320:124374. https://doi.org/10.1016/J.BIORTECH.2020.124374
Zhang L, Tan J, Xing G et al (2021) Cotton stalk-derived hydrothermal carbon for methylene blue dye removal: investigation of the raw material plant tissues. Bioresour Bioprocess 8:1–11. https://doi.org/10.1186/s40643-021-00364-8
Li F, Zimmerman AR, Hu X et al (2020) One-pot synthesis and characterization of engineered hydrochar by hydrothermal carbonization of biomass with ZnCl2. Chemosphere 254:126866. https://doi.org/10.1016/J.CHEMOSPHERE.2020.126866
Hessien M (2022) Microwave-assisted hydrothermal carbonization of pomegranate peels into hydrochar for environmental applications. Energies 15. https://doi.org/10.3390/en15103629
Funding
This work received financial support of China Scholarship Council (CSC) through PhD fellowship.
Author information
Authors and Affiliations
Contributions
Xin Li: sample preparation, material characterization, redaction of the manuscript under supervision of Pavel Kuzhir and Charlotte Hurel. Elemental analysis was performed by Erik Bonjour and Patrick Jame. All the authors read, commented, corrected the previous versions of the manuscript, and finally approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
No human or animal subjects were used in this research.
Consent to participate
All authors agreed with being involved in the research project.
Consent for publication
All authors have read and agree to the content submitted.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 802 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Bonjour, E., Jame, P. et al. Production of hydrochar from biomass waste as economical adsorbents for methylene blue—insight of occurring adsorption phenomena. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04122-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04122-y