Abstract
Separating amphiphilic compounds from complex matrix is challenging. Surfactin, cyclic lipopeptides group, was used as a model to find the proper separation conditions and parameters. The aim of the work was to solve the emulsification plug problem in countercurrent partition chromatography (CPC). The selected solvent system was a composition of n-heptane/n-butanol/methanol/aqueous buffer (20 mM disodium phosphate with 50 mM NaCl) at a ratio of 2:3:2:3. Several elution method modifications were applied. The most important was an appropriate mobile phase flow rate adjustment (flow rate gradient), minimizing the risk of stationary phase leakage. During the CPC procedures, the hydrostatic pressure was monitored as a factor of stationary phase retention. The hydrostatic pressure indicated the biphasic system’s stability. A flow rate of 2 ml/min resulted in column stabilization and peak resolution during chromatographic separation. In order to avoid uncontrolled leakage, the flow rate was increased gradually. At 3.5 ml/min, there was a small loss of the stationary phase as the hydrostatic pressure decreased from 32 to nearly 18 bar. After designing the method, it was tested with SU containing extracts obtained from SSF to show its effectiveness. Our results showed that the single dual-mode method can be used to remove matrix’s impurities and separate surfactin as a mixture of homologues with more than 80% purity. Moreover, application of the double dual-mode method allows for the isolation of fractions containing individual surfactin homologues in amounts of about 70% of the sample, which makes it possible to conduct further biological research.
Similar content being viewed by others
1 Introduction
The purification of natural products from solid state fermentation process (SSF) is a very demanding and complicated task due to the necessity of solid phase extraction and handle with complicated sample matrix. An example of such compounds are cyclic lipopeptides (CLP) which are biosurfactants (BS) of increasing interest to scientists and industrial manufacturers. One of the most known CLP is surfactin (SU) produced by some Bacillus subtilis strains. It is a group of compounds based on heptapeptide ring (ELVLDLL) closed with a lactone bond by β-hydroxyl fatty acid. Similar to the other CLP, SU is a secondary metabolite produced by nonribosomal peptide synthetases (NRPS) so it constitutes wide group of structures [1]. Its diversity relies on different numbers of carbon atoms and branching in the fatty acid part of the molecule (n-, iso-, and anteiso- forms). Additionally, a different substitution of amino acids in the ring is possible, but the chirality pattern (LLDLLDL) is always unchanged [2, 3]. Due to its surface activity, emulsyfing properties, biocompability, and biological activities as an antitumor, antiviral, and antifungal, it has been used to stabilize carrier biosystems [4,5,6]. The best known and widely used method of surfactin production is submerged fermentation (SmF) which has numerous described isolation methods [7, 8]. Nevertheless, the SSF appears to be a better choice for the production. It gives much higher yields of secondary metabolites, allows the use of agricultural waste (such as rapeseed meal, molasses, or oat husk) as substrates, and is a much cheaper and less complicated process to carry out than SmF [9]. However, an unsolved problem is the isolation of pure surfactin from the fermented biomass. The crude product has complicated matrix and constitutes the minority of the sample. In such a situation, commonly used methods like preparative HPLC cannot be used due to the silica resign containing columns’ limitations.
Counter-current chromatography (CCC) increasingly solves that problem [10, 11]. Centrifugal partition chromatography (CPC) is a hydrostatic variant of CCC, where stationary phase retention is maintained by a centrifugal field [12]. As stationary and mobile phases in CPC are a system of two immiscible liquids, there is no need to exploit typically used solid packed columns. They are replaced by a rotor consisting in small cells connected in series, where one such cell filled with a biphasic liquid system constitutes a physically existing theoretical plate [13]. The rotor content can be easily replaced with new phases so this solution allows to avoid any limitations of solid packed columns. Firstly, injection of highly contaminated samples with a complex composition without risk of damage, blockage, or contamination of the chromatographic resign is possible. Moreover, there is no irreversible adsorption of the analyte and therefore product loss is not a problem. Thirdly, the rotor can be filled with any system of two immiscible liquids designed on the basis of the analyte’s properties, so there is no need to purchase special dedicated columns [14]. In addition, by selecting the correct size of the rotor, the method can be scaled-up [15]. Ultimately, CPC is low solvent consuming compared to other preparative-scale chromatographic methods [16]. All these advantages result in several solutions based on CPC, for example, isolation of xylindein produced by Chlorociboria auruginosa [17], monosacharides from hydrolyzed sugar beet pulp [18], flavonoids and stilbenoids from Parthenocissus tricuspidata [16], geniposide from Gardenia jasminoides [19], or n-alkylresorcinols from wheat bran [20].
BS which are often present in natural matrixes exhibit emulsifying properties, which negatively affects the stability of the biphasic system in countercurrent chromatography. In CPC stationary, phase retention inside the rotor is maintained by a centrifugal field. The classic method of improving stationary phase retention consists in decreasing the flow rate and increasing the rotational speed. As a result, the stationary phase becomes more controllable, but the separation time significantly increases [21]. The main difficulty in optimizing CPC separation conditions of amphiphilic compounds is to find a proper solvent system, and the problem lies in the different partition coefficients (KD) of the substances to be separated. According to Martin and Synge [22,23,24], the peaks of the separated substances should be mathematically assigned to their partition coefficients. The ratios of KD values for neighboring peaks (known as α coefficients) in the simple CCC procedure should exceed 1.5 [25]. Nevertheless, the separation of chromatographically neighboring compounds with α lower than 1.5 is possible if the dual-mode method for CCC/CPC proposed by Bruening et al. [26] or the multiple dual-mode method [27] is used. The (multiple) dual-mode method involves the inversion of the separation modes at a certain moment of the procedure. After such inversion stationary phase becomes a new mobile phase and the mobile phase becomes a new stationary phase so the basic mode of operation reverses. The (multiple) dual-mode procedure can increase the number of theoretical plates for better separation and sometimes is the only way to separate the nearest peaks of the desired analytes [27]. In case of BS separation the amphiphilic properties can cause an emulsification sample plug [28], which may lead to a complete “wash-out” of the stationary phase without any separation. The most common solution is to decrease the flow rate of the mobile phase and increase the rotational speed in order to separate the phases in a stronger centrifugal field. Marchal et al. [20] demonstrated that by using the pseudo-ternary diagram it is often possible to find some system stability limitations after sample injection (e.g., the amount of the injected sample) before starting the CPC procedure.
In this publication, CPC is presented as an effective tool to isolate pure surfactin from SSF production. Firstly, we adopted surfactin as a model biosurfactant in order to find the solution to the emulsification plug problem, which included appropriate optimization of flow rate and centrifugal field. The second aim of this research was to show CPC as a method able to purify BS from complicated mixtures and to separate its particular isoforms with satisfactory purities. We developed a new method to separate amphiphilic compounds from complex matrixes using CPC with Bacillus subtilis surfactin as a model.
2 Materials and methods
2.1 Materials
HPLC gradient-grade methanol and acetonitrile were purchased from Merck (Chromasolv). Methanol, n-butanol, n-heptane, other analytical-grade solvents for CPC, and salts (NaCl and Na2HPO4*12H2O) were purchased from Chempur (Tarnowskie Góry, Poland). Surfactin standard and SSF extracts for purification tests were obtained as a gift from the InventionBio company. The water used was deionized using the HLP104V Hydrolab reverse-osmosis system (Wiślina, Poland).
2.2 Surfactin extraction from fermented biomass
The biomass from SSF process was obtained as a gift from InventionBio SA biorefinery. In the first step it was extracted with 0.1 M NaHCO3(aq) and centrifuged. Then the supernatant was collected and the extraction step was repeated. Combined extracts were acidified with 6 M hydrochloric acid to pH=2 and left at 4°C overnight to precipitate all the surfactin. Next day the solid residue was isolated after centrifugation and freeze-dried. The obtained solid, brown crude product was analyzed with HPLC-UV-MS in order to identify surfactin and measure its content. Then sample was injected directly for the purification with CPC.
2.3 High-performance liquid chromatography
Surfactin was analyzed using HPLC-UV-MS system (AcquityArc, Waters) equipped with a Cortex C18 column (4.6 × 50 mm, 2.7 μm). The column temperature was set to 40°C and samples were kept at 15°C. The mobile phase consisted of acetonitrile and water both with addition of formic acid (0.1% volume). The separation method was 10 min long, starting from 1:1 (v/v) mixture with increasing acetonitrile gradient and flow rate 1.0 ml/min. The quantitative detection was conducted with UV lamp set to 207 nm. For the homologue identification, surfactin was analyzed with an additional single quadrupole mass detector. Then m/z values and retention times were compared with the standard and used to distinguish the homologues based on their molecular masses.
2.3.1 Analysis of extracts and fractions
The SSF extracts were analyzed before the CPC procedure in order to determine the surfactin content. For this purpose, 5 mg of crude product was dissolved in 2 ml of MeOH and shaken for 30 min at room temperature. Then the suspension was centrifuged (13.4k rpm, 5 min), filtered through a syringe filter (0.22 μm) and injected to the HPLC analysis. Quantitative analysis was done based on the external standard. Every fraction isolated during the CPC procedure was analyzed in the following way: 500 μL aliquot was evaporated, suspended in 1 ml of methanol and filtered through a 0.22-μm syringe filter. Then 10 μL were injected for analysis.
2.4 Pseudo-ternary diagram
To prove the applicability of the selected biphasic system, a phase diagram was prepared. Two experiments were carried out for its preparation. In the first one, 1 ml surfactin solutions in the lower phase (mass concentrations: 2, 5, 7, 12, 15, 17, 20, 25%) were prepared and titrated with the upper phase, using 50 μl aliquots. The second experiment consisted in preparing the same surfactin concentrations in the upper phase and titrating them with the lower phase (using the same aliquots). All the solutions were placed in glass tubes equipped with plastic stoppers (to avoid changes in solvent composition through evaporation). The titration was conducted until the first biphasic system appeared. The experimental results were used to approximate the solubility isotherm (the dashed line) in the Gibbs triangle diagram. The blue points in the diagram represent the experimental data. The experiments were carried out under standard conditions (289.15 K, 105 Pa). After the diagram was prepared, two points were marked — the highest and the lowest possible surfactin concentration inside the rotor during the procedure.
2.5 Centrifugal partition chromatography
2.5.1 Apparatus
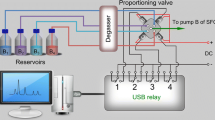
Spot CPC apparatus made by Gilson Glider was used for CPC. Each item of the apparatus had two separate rotors (1000 ml and 250 ml) and was equipped with the PLC21250 preparative chromatography system with an integrated control module together with the Armen Glider 5.1 CPC software. The 250 ml rotors were used in the experiments described below. The integrated detectors were 254 diode DADs (200-600 nm).
2.5.2 Determination of solvent system for CPC
About 40 solvent systems were tested: starting with the Arizona range (including hexane), followed by systems with n-butanol and ending with systems with dichloromethane (see supplementary data SM1). All the systems were tested using the traditional shake-flask method and a surfactin concentration of 1 mg/ml (calculated for the whole system volume). After many experiments only the systems with salts and buffers were found to be useful. In order to determine the upper phase/lower phase partition coefficients of the surfactin analogues, the following experiment was done. A surfactin sample (10 mg) was dissolved in 5 ml of the lower phase. Then 1 ml of this solution was mixed with 1 ml of the upper phase and left for the two phases to separate. 500 μl of each of the phases was isolated and evaporated. The remaining solid was dissolved in methanol, filtered and analyzed using HPLC. The areas under the peaks of the surfactin analogues provide information about the partition ratio.
The final system consisted of a phosphate-saline buffer, methanol, butanol and heptane at a ratio of 3:2:3:2 (v/v/v/v). The phosphate-saline buffer was made of 20 mM of alkali disodium phosphate and 50 mM of sodium chloride. When the system was ready, the pH of the lower (water-containing) phase was close to 7.5.
2.5.3 Column preparation
The column preparation program is shown in Table 2. Rotor filling was conducted at a rotational speed of 500 rpm and a flow rate of 30 ml/min. Stationary phase equilibration was performed at 2000 rpm and 8 ml/min. As a result of the equilibration with the mobile phase, the flow gradient decreased from 8 to 2 ml/min. The flow rate of 2 ml/min was maintained for a few minutes to stabilize the system. The flow rate reduction was necessary to bring the column to the initial (elution) conditions.
2.5.4 Sample preparation
Injection was effected through an injection valve, using a 5-ml sample loop. The loop was initially filled with the chosen solvent system, using the upper phase and the lower phase at a ratio of 1:1 (v/v). The sample was dissolved in the solvent system to a final concentration of 40 mg/ml (in the system) and filtered. The total injection volume amounted to 5 ml (2.5 ml of the upper phase and 2.5 ml of the lower phase). In the case of SSF extracts, 600 mg of crude product was suspended in 5 ml of the solvent system 1:1 (v/v), vortexed for 30 s, centrifuged (8k rpm, 3 min) and filtered through a 0.22-μm nylon syringe filter. The filtered solution was injected.
2.5.5 Single dual-mode & double dual-mode procedures
Since the differences between the partition coefficients were not ideal, the dual-mode method procedures were applied. Single dual-mode elution was used in the program shown in Table 2. The single dual-mode procedure involves the simple dual-mode method with a single switching of the ascending/descending (ASC/DSC) valve during elution. Double dual-mode elution was performed using a program listed also in Table 2. The double dual-mode procedure involves a double switching of the ASC/DSC valve during elution and has been confirmed by several independent experiments (see supplementary data SM2). In both cases, the 207-nm wavelength was monitored.
3 Results and discussion
3.1 CPC as a solution for complicated samples
The procedures published so far for the isolation of biosurfactants from fermented biomass are mainly analytical procedures based on methanol extraction [29,30,31,32]. In the case of preparative methods, extraction with methanol is only the first stage, followed by purification with activated carbon, recrystallization from an acetone/water system and injection into a chromatographic C18 column. Then the isolated fractions are subjected to further purification stages [33]. Other methods describe ethanol extraction and direct injection of crude extract to a C18 column but there is no data about the purity of separated surfactin [34]. As another example, C. T. Slivinsky et. al. published a strategy for the isolation of surfactin for analytical purposes. The biomass was extracted with water and then this extract was acidified in order to precipitate crude surfactin. The crude product was dissolved in distilled water and extracted with a mixture of chloroform and methanol to give a purified sample subjected to HPLC analysis [35]. Based on this method, the extraction with base addition (NaHCO3) was performed and then the crude surfactin was precipitated. The extract obtained from SSF product was a brown, sticky powder with 20% w/w surfactin content, which means that impurities constitute the majority of the sample. Due to the limitations of silica-packed columns (such as irreversible adsorption or possibility of damage and blockage), preparative HPLC cannot be used for this type of analytes without additional purification steps. Therefore CPC, a variant of liquid-liquid chromatography, appears to be the solution. However, amphiphilic substances significantly decrease interfacial tension, which results in emulsification of the mobile and stationary phases. Unlike the case of non-surfactants (for which the CPC method is typically used), the injected concentration of surfactants is significantly lower and leads to the complete “wash-out” of the stationary phase due to sample emulsification and plugging. This applies also to samples in which surface-active compounds are present, making stationary phase retention difficult. As a result, the purification and separation of the other substances is difficult and sometimes simply impossible. This is why the typical separation conditions for the Gilson 250-ml CPC modules (recommended by the manufacturer) are inappropriate for surfactants. In the case of emulsifying compounds (for example surfactin), the flow rate and injection volume should be decreased and the rotational speed increased, so these parameters had to be optimized.
3.2 Characteristics of the selected biphasic system
The HPLC chromatogram of the mixture of C12, C13, C14, C15 and C16 homologues (Fig. 1) shows at least 2 peaks for each of the homologues (for their various isoforms). According to Y. Zhao et al. [36], there can exist three isoforms (n-, iso-, and anteiso-) for each of the homologues. Using the HPLC method, one can separate the individual isoforms, but one is unable to assign the particular peaks to a specific geometry. Therefore, for research considerations, the isoforms of the individual homologues are distinguished on the chromatograms with symbols a, b, c as shown in Fig. 1. The partition coefficients for surfactin forms were determined using HPLC with UV detection (207 nm), and they are listed in Table 1. As expected, separation coefficients α are not ideal. The desired values should be about 1.5, while the coefficients obtained for the chosen biphasic system are approximately 1. However, the use of a switch in the flow direction during the separation procedure (multiple dual-mode) enables an increase in the resolution efficiency of the method in a way that compensates for the imperfect values of the α coefficients. Homologues with the longest and shortest chains (C16, C12 and C11) represent the smallest quantities in the surfactin mixture (Fig. 1) and so they were observed in the UV chromatograms but not recovered from the fractions.
In order to simulate the behavior of the biphasic system during the separation, two points have been shown on the prepared pseudo-ternary phase diagram — the highest and the lowest possible surfactin concentration. The point of the highest possible surfactin concentration is the composition of the injected sample. It was found by measuring volumes of the biphasic system’s lower and upper layers, which is marked on the diagram as a red square (Fig. 2). The lowest theoretical concentration of surfactin (which is not achieved during separation) is the result of its dissolution in the entire volume of the biphasic system (the rotor content). In order to find that point on the diagram, 245 ml of the two-phase mixture 1:1 (v/v) and 5 ml of the surfactin (200 mg) solution were placed in a graduated cylinder and mixed by shaking. Then the volumes of the phases were measured. The composition of the mixture is represented by the green square in the pseudo-ternary phase diagram (Fig. 2). It appears from the diagram that when the mass of the injected surfactin is 200 mg, full miscibility of the phases during the separation process cannot be achieved, which makes separation possible. Surfactin content in crude extract is 20% by mass, which means that one gram of SSF extract can be injected for the separation with a 250-mL rotor filled with the solvent system. In the case of example prep-HPLC procedure (preceded by a multi-stage purification) [34], there is no data about mass of the injected sample, but concluding from the used column, the maximum sample load is below 100 mg. In the case of the presented CPC method, the scale is ten times higher without additional purification steps. Moreover, compared to the other published similar scale CPC procedures [37, 38], the mass of injected extract is two times higher.
3.3 Single dual-mode method optimization
In the first experiment, the elution flow rate was reduced from the standard flow rate to 4 ml/min, but this resulted in the uncontrolled wash-out of the rotor content. The emulsion (a mixture of the mobile phase and the stationary phase) which formed made absorbance registration impossible and caused a complete wash-out of the biphasic system. This behavior is characteristic for the emulsification sample plug [39, 40]. However, it was not expected because only 5 ml of the surfactin solution was injected (the surfactin concentration amounted to 40 mg/ml), which is a rather small quantity for a CPC column [20, 41]. A typical injection volume constitutes 10% of the entire rotor capacity, while in our case it was 2%. The flow rate in the final stage of column preparation was set to 4 ml/min and rotational speed was increased to 2000 rpm (from the initially used 1600 rpm). After the first experiment, the method was changed, including column preparation and elution. The column preparation program shown in Table 2 was used in the further experimentation. The flow rate was decreased to 2 ml/min in the initial stage and then gradually increased during elution.
The single dual-mode elution (and extrusion) program is shown in Table 2. It involves single switching of the ascending/descending (ASC/DSC) valve during elution. The chromatogram obtained is shown in Fig. 3. Surprisingly, there is a much higher affinity for the lower (aqueous) phase than the partition coefficients suggest. This was probably caused by micellization since surfactin may have a higher ability to create regular micelles in aqueous conditions rather than reversed micelles (which can be formed in organic nonpolar systems) [42].
The hydrostatic pressure behavior is shown in Fig. 3 as the indicator for the biphasic system’s stability. Flow rate gradients from 2 to 3.5 ml/min were used, with no leakage of the stationary phase up to nearly 3 ml/min as no significant loss of hydrostatic pressure occurred. When the flow rate was increased to 3.5 ml/min, there was a small loss of the stationary phase as the hydrostatic pressure decreased from c.a. 32 to nearly 18 bar. This slight gradient of the flow rate helped to push the surfactin through the column, widening the injection zone volume. The selected flow rate of 3.5 ml/min enabled the control of stationary phase flooding. The two advantages of the controlled small leakage (of the stationary phase) are (i) a smaller volume of the sample moves through the column (the injection zone volume is tighter) and (ii) there is more mobile phase, which becomes a stationary phase after the ASC/DSC valve is switched, enabling better separation. After the ASC/DSC valve was switched, the hydrostatic pressure initially increased and then decreased gradually to a stable point during the elution of the surfactin. Finally, the single dual-mode method, which allows the separation of surfactin as a mixture of homologues, was optimized. Such separation was conducted in reasonable time with low solvent consumption compared to other similar scale CPC procedures [37, 43, 44].
3.4 Double dual-mode method optimization
Double dual-mode elution was performed using the program listed in Table 2. The double dual-mode procedure involves a double switching of the ASC/DSC valve during elution. The DDM chromatogram, the flow rate and pressure measurements are presented in Fig. 3. The first few steps were identical to those used in the SDM procedure, except that the ASC/DSC valve was switched a second time during the elution, enabling another ASC mode. During this second ASC mode, a further flow rate gradient was applied. The flow rate was increased from 3.0 to 6.0 ml/min at the end of the elution. This helped to elute the substances in a reasonable time. The elution would have lasted 2 h longer if the flow rate had not been increased through this gradient.
Interestingly, during this gradient step there was no significant flooding of the stationary phase as the pressure decreased merely from 35 to 30 bar. This can be explained by the lowered local surfactin concentration due to the separation of the homologues. The flow rate being increased up to 6 ml/min at the end of the double dual-mode procedure did not cause any significant stationary phase flooding, and separation was achieved in a reasonable time.
Application of double dual-mode made the surfactin peak much wider than in the single dual-mode method to such a degree that this peak can be split into fractions containing pre-purified individual surfactin homologues.
3.5 Application for SSF extract
After finding the proper conditions with use of pure surfactin as a model, the designed method was tested with SSF extract. After injection and purification with the use of DDM program, eluted fractions were collected and analyzed with HPLC. The experiment results showed potential to isolate particular homologues. The collected fractions differed significantly in the quantitative ratio of surfactin homologues. The first to elute were the hydrophobic C16 and C15 ones, where the C16 homologue yield was very small due to its low content in the injected sample. The next homologues leaving the rotor were C14, C13, and C12 (similar to C16 in very small quantity). The main components C15, C14, and C13 accounted for 60–70% of the fractions from which they were measured. Such purity allows for further separation using preparative HPLC in order to obtain 100% surfactin homologues, which are still not fully researched compounds. The chromatograms of selected fractions are shown in Fig. 4. Then to determine the overall surfactin content in the eluted product, all the fractions were mixed together and evaporated to dryness. The solid residue after dissolving in ethyl acetate was filtered through a short silica plug in order to reduce buffer salt content. Then the solution was evaporated to dryness giving a product with 80% purity (determined with HPLC).
4 Conclusion
Our research has demonstrated that difficulties connected with samples’ complexity and the presence of amphiphilic substances in CPC use can be solved. We used surfactin — a biosurfactant naturally produced by Bacillus subtilis, having classic amphiphilic properties, as a model.
We have shown that the risk of stationary phase leakage can be significantly reduced if there is no large injection (its volume was reduced from the typically used 10 to 2% of the rotor capacity). But this also depends on the elution program. If the flow rate is decreased, and rotational speed increased, there is a higher chance for the stationary phase to be retained inside the column. Finally, flow rate was set to 2 ml/min and the rotational speed to 2000 rpm (while the standard conditions suggested by the producer are 5–8 ml/min and 800 rpm). Such low flow rate makes the method time-consuming, so a simple gradient was introduced. Slow increase of the flow rate from 2 to 3.5 ml/min resulted in reasonable separation time and no loss of stationary phase. The designed and tested methods turned out to be effective tools to isolate surfactin from complex mixtures of natural origin, and to separate its homologues with satisfactory purity. The injected extract contained 20% of surfactin by mass while the isolated product had more than 80% purity. Moreover, collection of the single fractions allows to obtain C13, C14, and C15 homologues with purity of about 70% (compared to the other homologues), which makes it possible for further research or purification. Our results show the first use of CPC in biosurfactant isolation after SSF process and give a solution to the problem of emulsification of the phases involved in the separation. In addition, despite the problem with phase emulsification, the developed methods have satisfactory performance compared to other published applications of the CPC technique for natural product separation [37, 43, 45, 46]. The presented chromatographic technique showed significant advantages over the typical preparative chromatography. No need for additional sample purification before injection and the possibility of separating such a large sample in one experiment make it much easier, faster and cheaper to operate. In addition, the ease of scalability of the CPC technique opens the way to research on increasing the scale of surfactin purification. In addition, our results may be the basis for the application of the CPC technique in the purification of other biosurfactants.
Data availability
Not applicable.
References
Roongsawang N, Washio K, Morikawa M (2010) Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int J Mol Sci 12:141–172. https://doi.org/10.3390/ijms12010141
Bonmatin J-M, Labbé H, Grangemard I et al (1995) Production, isolation and characterization of [Leu4]- and [Ile4] surfactins from Bacillus subtilis. Lett Pept Sci 2:41–47. https://doi.org/10.1007/BF00122922
Liu JF, Yang J, Yang SZ et al (2012) Effects of different amino acids in culture media on surfactin variants produced by Bacillus subtilis TD7. Appl Biochem Biotechnol 166:2091–2100. https://doi.org/10.1007/s12010-012-9636-5
Lewińska A, Domżał-Kędzia M, Maciejczyk E et al (2021) Design and engineering of “green” nanoemulsions for enhanced topical delivery of bakuchiol achieved in a sustainable manner: a novel eco-friendly approach to bioretinol. Int J Mol Sci 22:10091. https://doi.org/10.3390/ijms221810091
Lewińska A (2021) Optimizing the process design of oil-in-water nanoemulsion for delivering poorly soluble cannabidiol oil. Processes 9:1180. https://doi.org/10.3390/pr9071180
Lewińska A, Domżał-Kędzia M, Jaromin A, Łukaszewicz M (2020) Nanoemulsion stabilized by safe surfactin from Bacillus subtilis as a multifunctional, custom-designed smart delivery system. Pharmaceutics 12:1–21. https://doi.org/10.3390/pharmaceutics12100953
Klausmann P, Hennemann K, Hoffmann M et al (2021) Bacillus subtilis High cell density fermentation using a sporulation-deficient strain for the production of surfactin. Appl Microbiol Biotechnol 105:4141–4151. https://doi.org/10.1007/s00253-021-11330-x
Biniarz P, Coutte F, Gancel F, Łukaszewicz M (2018) High-throughput optimization of medium components and culture conditions for the efficient production of a lipopeptide pseudofactin by Pseudomonas fluorescens BD5. Microb Cell Fact 17:1–18. https://doi.org/10.1186/s12934-018-0968-x
Viniegra-Gonzàlez G (1997) solid state fermentation: definition, Characteristics, Limitations and Monitoring. Adv Solid State Ferment:5–22. https://doi.org/10.1007/978-94-017-0661-2_2
Yoon KD, Chin Y-W, Kim J (2010) Centrifugal partition chromatography: application to natural products in 1994–2009. J Liq Chromatogr Relat Technol 33:1208–1254. https://doi.org/10.1080/10826076.2010.484374
Foucault AP, Chevolot L (1998) Counter-current chromatography: instrumentation, solvent selection and some recent applications to natural product purification. J Chromatogr A 808:3–22. https://doi.org/10.1016/S0021-9673(98)00121-6
Berthod A, Maryutina T, Spivakov B et al (2009) Countercurrent chromatography in analytical chemistry (IUPAC technical report). Pure Appl Chem 81:355–387. https://doi.org/10.1351/PAC-REP-08-06-05
Skalicka-Woźniak K, Garrard I (2015) A comprehensive classification of solvent systems used for natural product purifications in countercurrent and centrifugal partition chromatography. Nat Prod Rep 32:1556–1561. https://doi.org/10.1039/c5np00061k
Pan Y, Lu Y (2007) Recent progress in countercurrent chromatography. J Liq Chromatogr Relat Technol 30:649–679. https://doi.org/10.1080/10826070701190948
Sutherland IA (2007) Recent progress on the industrial scale-up of counter-current chromatography. J Chromatogr A 1151:6–13. https://doi.org/10.1016/j.chroma.2007.01.143
Jeon JS, Kim CY (2013) Preparative separation and purification of flavonoids and stilbenoids from Parthenocissus tricuspidata stems by dual-mode centrifugal partition chromatography. Sep Purif Technol 105:1–7. https://doi.org/10.1016/j.seppur.2012.11.010
Boonloed A, Weber GL, Ramzy KM et al (2016) Centrifugal partition chromatography: a preparative tool for isolation and purification of xylindein from Chlorociboria aeruginosa. J Chromatogr A 1478:19–25. https://doi.org/10.1016/j.chroma.2016.11.026
Ward DP, Cárdenas-Fernández M, Hewitson P et al (2015) Centrifugal partition chromatography in a biorefinery context: separation of monosaccharides from hydrolysed sugar beet pulp. J Chromatogr A 1411:84–91. https://doi.org/10.1016/j.chroma.2015.08.006
Kim CY, Kim J (2007) Preparative isolation and purification of geniposide from gardenia fruits by centrifugal partition chromatography. Phytochem Anal 18:115–117. https://doi.org/10.1002/pca.958
Marchal L, Intes O, Foucault A et al (2003) Rational improvement of centrifugal partition chromatographic settings for the production of 5-n-alkylresorcinols from wheat bran lipid extract: I. Flooding conditions—optimizing the injection step. J Chromatogr A 1005:51–62
Bojczuk M, Żyżelewicz D, Hodurek P (2017) Centrifugal partition chromatography - a review of recent applications and some classic references. J Sep Sci 40:1597–1609. https://doi.org/10.1002/jssc.201601221
Martin AJP, Synge RLM (1977) A new form of chromatogram employing two liquid phases. Trends Biochem Sci 2:N245. https://doi.org/10.1016/0968-0004(77)90204-3
Butt WR, Henly AA, Morris CJOR (1948) The polarographic estimation of steroid hormones. Biochem J 42:447–452. https://doi.org/10.1042/bj0420447
Martin AJ, Synge RL (1941) Separation of the higher monoamino-acids by counter-current liquid-liquid extraction: the amino-acid composition of wool. Biochem J 35:91–121. https://doi.org/10.1042/bj0350091
Berthod A, Billardello B, Geoffroy S (1999) Polyphenols in countercurrent chromatography. An example of large scale separation. Analusis 27:750–757. https://doi.org/10.1051/analusis:1999140
Bruening RC, Oltz EM, Furukawa J et al (1986) Isolation of tunichrome B-1, A Reducing blood pigment of the sea squirt, Ascidia Nigra. J Nat Prod 49:193–204. https://doi.org/10.1021/np50044a001
Delannay E, Toribio A, Boudesocque L et al (2006) Multiple dual-mode centrifugal partition chromatography, a semi-continuous development mode for routine laboratory-scale purifications. J Chromatogr A 1127:45–51. https://doi.org/10.1016/j.chroma.2006.05.069
Berthod A, Brown L, Leitão GG, Leitão SG (2002) Chapter 2 Operating a countercurrent chromatography machine. In: Berthod A (ed) Countercurrent chromatography. Elsevier, pp 21–47. https://doi.org/10.1016/S0166-526X(02)80005-8
Ohno A, Ano T, Shoda M (1995) Effect of temperature on production of lipopeptide antibiotics, iturin A and surfactin by a dual producer, Bacillus subtilis RB14, in Solid-State Fermentation. J Ferment Bioeng 80:517–519
Ohno A, Ano T, Shoda M (1995) Production of a lipopeptide antibiotic, surfactin, by recombinant Bacillus subtilis in solid state fermentation. Biotechnol Bioeng 47:209–214. https://doi.org/10.1002/bit.260470212
Ohno A, Ano T, Shoda M (1993) Production of the antifungal peptide antibiotic, iturin by Bacillus subtilis NB22 in solid state fermentation. J Ferment Bioeng 75:23–27. https://doi.org/10.1016/0922-338X(93)90172-5
Zhu Z, Zhang F, Wei Z et al (2013) The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J Environ Manag 127:96–102. https://doi.org/10.1016/j.jenvman.2013.04.017
Nakayama S, Takahashi S, Hirai M, Shoda M (1997) Isolation of new variants of surfactin by a recombinant Bacillus subtilis. Appl Microbiol Biotechnol 48:80–82. https://doi.org/10.1007/s002530051018
Kim KM, Lee JY, Kim CK, Kang JS (2009) Isolation and characterization of surfactin produced by Bacillus polyfermenticus KJS-2. Arch Pharm Res 32:711–715. https://doi.org/10.1007/s12272-009-1509-2
Slivinski CT, Mallmann E, De Araújo JM et al (2012) Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent. Process Biochem 47:1848–1855. https://doi.org/10.1016/j.procbio.2012.06.014
Zhao Y, Yang SZ, Mu BZ (2012) Quantitative analyses of the isoforms of surfactin produced by Bacillus subtilis HSO 121 Using GC-MS. Anal Sci 28:789–793. https://doi.org/10.2116/analsci.28.789
Renault JH, Thepenier P, Zeches-Hanrot M, Foucault AP (1995) Separation of the two major anthocyanins from champagne vintage by-products by gradient elution centrifugal partition chromatography. J Liq Chromatogr 18:1663–1670. https://doi.org/10.1080/10826079508009303
Kim SM, Shang YF, Um BH (2010) Preparative separation of chlorogenic acid by centrifugal partition chromatography from highbush blueberry leaves vaccinium corymbosum L. Phytochem Anal 21:457–462. https://doi.org/10.1002/pca.1218
Matsuda K, Matsuda S, Ito Y (1998) Toroidal coil counter-current chromatography: achievement of high resolution by optimizing flow-rate, rotation speed, sample volume and tube length. J Chromatogr A 808:95–104. https://doi.org/10.1016/S0021-9673(98)00114-9
Menges RA, Menges TS, Bertrand GL et al (1992) Extraction of nonionic surfactants from waste water using centrifugal partition chromatography. J Liq Chromatogr 15:2909–2925. https://doi.org/10.1080/10826079208016356
Toribio A, Nuzillard JM, Renault JH (2007) Strong ion-exchange centrifugal partition chromatography as an efficient method for the large-scale purification of glucosinolates. J Chromatogr A 1170:44–51. https://doi.org/10.1016/j.chroma.2007.09.004
Liu Y, Kuang P, Guo S et al (2018) An overview of recent progress in solvent systems, additives and modifiers of counter current chromatography. New J Chem 42:6584–6600. https://doi.org/10.1039/c7nj04747a
Kim BR, Thapa P, Kim HM et al (2020) Purification of phenylpropanoids from the scaly bulbs of lilium longiflorum by CPC and determination of Their DPP-IV Inhibitory Potentials. ACS Omega 5:4050–4057. https://doi.org/10.1021/acsomega.9b03649
Ward DP, Hewitson P, Cárdenas-Fernández M et al (2017) Centrifugal partition chromatography in a biorefinery context: optimisation and scale-up of monosaccharide fractionation from hydrolysed sugar beet pulp. J Chromatogr A 1497:56–63. https://doi.org/10.1016/j.chroma.2017.03.003
Kim CY, Kim J (2007) Preparative isolation and purification of geniposide from Gardenia fruits by centrifugal partition chromatography. Phytochem Anal 18:115–117. https://doi.org/10.1002/pca.958
Young Kim C, Ahn MJ, Kim J (2006) Preparative isolation of mangiferin from Anemarrhena asphodeloides rhizomes by centrifugal partition chromatography. J Liq Chromatogr Relat Technol 29:869–875. https://doi.org/10.1080/10826070500531391
Funding
Authors received financial support from the statutory activity of subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry and Faculty of Biotechnology of the University of Wroclaw. Work partially financed by POIR.01.01.02-00-0128/16-00 and POIR.02.01.00-00-0159/15-00.
Author information
Authors and Affiliations
Contributions
Conceptualization: AL., MŁ.; formal analysis: MB,.PH, AL.; investigation: MB.,PH, AL,; resources: AL, MŁ; writing—original draft preparation: MB, AL; writing—review and editing: all the authors; supervision: AL MŁ; funding acquisition: AL, MŁ. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
- Separation of surfactin by centrifugal partition chromatography.
- Solution of the emulsification sample plug problem.
- Influence of the flow rate on sample emulsification.
- Controlled flooding of the stationary phase.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bochynek, M., Hodurek, P., Łukaszewicz, M. et al. Centrifugal partition chromatography as a potential method of isolation and purification of amphiphilic substances from a solid-state fermentation process. Biomass Conv. Bioref. 13, 16333–16343 (2023). https://doi.org/10.1007/s13399-023-03821-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03821-w