Abstract
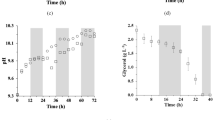
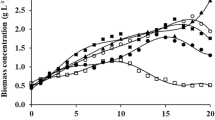
Glycerol is a byproduct of biodiesel production. Purified, this compound has several applications; however, in its crude form, its commercial value is limited. An alternative use of crude glycerol is its application as an organic carbon source in microalgal cultures. This study aimed to investigate the different concentrations in crude glycerol influence productivity, biomass concentration, and biomolecules content in cultures of Spirulina sp. LEB 18. Microalga was cultured in media supplemented with different glycerol concentrations in Erlenmeyer bioreactors during 15 days. The best glycerol concentration was 2.5 g L−1, which led to the maximum biomass productivity (0.43 g L day−1). Proteins were the largest component of the biomass (75.7% w w−1, 2.5 g L−1 glycerol) and could be used to obtain high-added-value compounds. Carbohydrates ranged from 7.0 (control) to 8.9% (1.5 g L−1) w w−1. Chlorophyll was reduced up to 47% (9.14 mg g−1) in the assay using 5 g L−1 of glycerol. The lipids produced in this condition (8.3% w w−1, 2.5 g L−1 glycerol) presented interesting properties for biodiesel production because of their high content of saturated fatty acids (260.56 mg g−1, 2.5 g L−1 glycerol). Microalgae culture with crude glycerol could aggregate value to a biodiesel co-product through biocompounds production making the biofuel production process more sustainable.



Similar content being viewed by others
References
Amigun B, Musango JK, Brent AC (2011) Community perspectives on the introduction of biodiesel production in the Eastern Cape Province of South Africa. Energy 36:2502–2508. https://doi.org/10.1016/j.energy.2011.01.042
Ministério de Minas e Energia (2018) Produção de biodiesel atinge 452 milhões de litros, maior volume nos últimos dez anos at http://www.mme.gov.br/web/guest/pagina-inicial/outras-noticas/-/asset_publisher/32hLrOzMKwWb/content/producao-de-biodiesel-atinge-452-milhoes-de-litros-maior-volume-nos-ultimos-dez-anos
Cavalheiro JMBT, Raposo RS, Almeida MCMD, Cesário MT, Sevrin C, Grandfils C, Fonseca MMR (2012) Effect of cultivation parameters on theproduction of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) andpoly(3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour Technol 111:391–397. https://doi.org/10.1016/j.biortech.2012.01.176\
Spoljaric IV, Lopar M, Koller M, Muhr A, Salerno A, Reiterer A, Malli K, Angerer H, Strohmeier K, Schober S, Mittelbach M, Horvat P (2013) Mathematical modeling of poly[(R)-3-hydroxyalkanoate] synthesis by Cupriavidus necator DSM 545 on substrates stemming from biodiesel production. Bioresour Technol 133:482–494. https://doi.org/10.1016/j.biortech.2013.01.126
Luo X, Ge X, Cui S, Li Y (2016) Value-added processing of crude glycerol into chemicals and polymers. Bioresour Technol 215:144–154. https://doi.org/10.1016/j.biortech.2016.03.042
Ma X, Zheng H, Addy M, Anderson E, Liu Y, Chen P, Ruan R (2016) Cultivation of Chlorella vulgaris in wastewater with waste glycerol: strategies for improving nutrients removal and enhancing lipid production. Bioresour Technol 207:252–261. https://doi.org/10.1016/j.biortech.2016.02.013
Morais EG, Druzian JI, Nunez IL, Morais MG, Costa JAV (2019) Glycerol increases growth, protein production and alters the fatty acids profile of Spirulina (Arthrospira) sp. LEB 18. Process Biochem 76:40–45. https://doi.org/10.1016/j.procbio.2018.09.024
Martinez N, Callejas N, Morais EG, Costa JAV, Jachmanian-Alpuy I, Vieitez-Osorio I (2017) Obtaining biodiesel from microalgae oil using ultrasound-assisted in-situ alkaline transesterification. Fuel 202:512–519. https://doi.org/10.1016/j.fuel.2017.04.040
Yen H, Hu C, Chen CY, Ho SH, Lee DJ, Chang JS (2013) Microalgae-based biorefinery: from biofuels to natural products. Bioresour Technol 135:166–174. https://doi.org/10.1016/j.biortech.2012.10.099
Garrido-Cardenas JA, Manzano-Agugliaro F, Acien-Fernandez FG, Molina-Grima E (2018) Microalgae research worldwide. Algal:50–60. https://doi.org/10.1016/j.algal.2018.08.005
Jesus CS, Uebel LS, Costa SS, Miranda AL, Morais EG, Morais MG, Costa JAV, Nunes IL, Ferreira ES, Druzian JI (2018) Outdoor pilot-scale cultivation of Spirulina sp. LEB 18 in different geographic locations for evaluating its growth and chemical composition. Bioresour Technol:86–94. https://doi.org/10.1016/j.biortech.2018.01.149
Morais MG, Radmann EM, Andrade MR, Teixeira GG, Brusch LRF, Costa JAV (2009) Pilot scale semicontinuous production of Spirulina biomass in southern Brazil. Aquaculture. 294:60–64. https://doi.org/10.1016/j.aquaculture.2009.05.009
Narayan MS, Manoj GP, Vatchravelu K, Bhagyalakshmi N, Mahadevaswamy M (2005) Utilization of glycerol as carbon source on the growth, pigment and lipid production in Spirulina platensis. Int J Food Sci Nutr 7:521–528. https://doi.org/10.1080/09637480500410085
Markou G, Kougia E, Kefalogianni I, Tsagou V, Arapoglou D, Chatzipavlidis I (2019) Effect of glycerol concentration and light intensity on growth and biochemical composition of Arthrospira (Spirulina) platensis: a study in semi-continuous mode with non-aseptic conditions. Appl Sci 9(21):4703. https://doi.org/10.3390/app9214703
Morais MG, Costa JAV, Marins LFF, Reichert CC, Dalcanton F, Durante AJ (2008) Isolation and caracterization of a new Arthrospira strain. Z Naturforsch C 63c:144–150
Zarrouk C (1966) Contribution à l’étude d’une cyanophycée. Influence de diveurs facteurs physiques et chimiques sur la croissance et photosynthese de Spirulina maxima. University of Paris, Paris
Costa JAV, Colla LM, Duarte Filho P (2004) Improving Spirulina platensis biomass yield using a fed-batch process. Bioresour Technol 92:237–241. https://doi.org/10.1016/j.biortech.2003.09.013
Morais MG, Costa JAV (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultived in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445
Ribeiro PLL, Silva ACMS, Menezes Filho JA, Druzian JI (2015) Impact of different by-products from the biodiesel industry and bacterial strains on the production, composition, and properties of novel polyhydroxyalkanoates containing achiral building blocks. Ind Crop Prod 69:212–223. https://doi.org/10.1016/j.indcrop.2015.02.035
Schimidell W, Lima AU, Aquarone E, Borzani W (2001) Biotecnologia Industrial, vol v. 2. São Paulo, Edgard Blücher LTDA
Bondioli P, Bella LD (2005) An alternative spectrophotometric method for the determination of free glycerol in biodiesel. Eur J Lipid Sci Technol 107:153–157. https://doi.org/10.1002/ejlt.200401054
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Dubois M, Gilles KA, Hamilton J, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Folch J, Lees M, Stanley GHA (1957) Simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 34:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Katiyar R, Gurjar BR, Bharti RK, Kumar A, Biswas S, Pruthi V (2017) Heterotrophic cultivation of microalgae in photobioreactor using low cost crude glycerol for enhanced biodiesel production. Renew Energy 113:1359–1365. https://doi.org/10.1016/j.renene.2017.06.100
Rym BD, Nejeh G, Lamia T, Ali Y, Rafika C, Khemissa G, Jihene A, Hela O, Hatem BO (2010) Modeling growth and photosynthetic response in Arthrospira platensis as function of light intensity and glucose concentration using factorial design. J Appl Phycol 22:745–752. https://doi.org/10.1007/s10811-010-9515-9
Deng X, Chen B, Xue C, Li D, Hu X, Gao K (2019) Biomass production and biochemical profiles of a freshwater microalga Chlorella kessleri in mixotrophic culture: Effects of light intensity and photoperiodicity. Bioresour Technol 273:358–367. https://doi.org/10.1016/j.biortech.2018.11.032
Morales-Sánchez D, Tinoco-Valencia R, Martinez A (2013) Heterotrophic growth of Neochloris oleoabundans using glucose as a carbon source. Biotechnol Biofuels:1–12. https://doi.org/10.1186/1754-6834-6-100
Neilson AH, Lewin RA (1974) The uptake and utilization of organic carbon by algae: an essay in comparative biochemistry. Phycologia 13:227–264. https://doi.org/10.2216/i0031-8884-13-3-227.1
Garcia OP, Escalante FME, Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res 45:11–36. https://doi.org/10.1016/j.watres.2010.08.037
Zheng H, Liu M, Lu Q, Wu X, Ma Y, Cheng Y, Addy M, Liu Y, Ruan R (2018) Balancing carbon/nitrogen ratio to improve nutrients removal and algal biomass production in piggery and brewery wastewaters. Bioresour Technol 249:479–486. https://doi.org/10.1016/j.biortech.2017.10.057
Paranjape K, Leite GB, Hallenbeck PC (2016) Effect of nitrogen regime on microalgal lipid production during mixotrophic growth with glycerol. Bioresour Technol 214:778–786. https://doi.org/10.1016/j.biortech.2016.05.020
Paranjape K, Leite GB, Hallenbeck PC (2016) Strain variation in microalgal lipid production during mixotrophic growth with glycerol. Bioresour Technol 204:80–88. https://doi.org/10.1016/j.biortech.2015.12.071
Salati S, Imporzano GD, Menin B, Veronesi D, Scaglia B, Abbruscato P, Mariani P, Adani F (2017) Mixotrophic cultivation of Chlorella for local protein production using. Bioresour Technol 230:82–89. https://doi.org/10.1016/j.biortech.2017.01.030
Nelson DL, Cox MM (2011) Princípios de Bioquimica de Lehninger, 5 ed., Sarvier.
El-Sheekh MM, Bedaiwy MY, Osman ME, Ismail MM (2012) Mixotrophic and heterotrophic growth of some microalgae using extract of fungal-treated wheat bran. Int J Recycling Org Waste Agric:1–12. https://doi.org/10.1186/2251-7715-1-12
Kadkhodaei S, Abbasiliasi S, Shun TJ, Masoumi HRF, Mohamed MS, Movahedi A, Rahime R, Ariff AB (2015) Enhancement of protein production by microalgae Dunaliella salina under mixotrophic condition using response surface methodology. RSC Adv 48:1–17. https://doi.org/10.1039/C5RA04546K
Morais EG, Cassuriaga APA, Callejas N, Martinez N, Vieitez-Osorio I, Jachmanian-Alpuy I, Santos LO, Morais MG, Costa JAV (2018) Evaluation of CO2 biofixation and biodiesel production by Spirulina (Arthospira) cultivated in air-lift photobioreactor. Braz Arch Biol Technol 61:1–11. https://doi.org/10.1590/1678-4324-2018161339
Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ Sci T Roy Soc Chem 2:759–766. https://doi.org/10.1039/B903941D
Song M, Pei H (2018) The growth and lipid accumulation of Scenedesmus quadricauda during batch mixotrophic/heterotrophic cultivation using xylose as a carbon source. Bioresour Technol 263:525–531. https://doi.org/10.1016/j.biortech.2018.05.020
Xue L, Chen H, Jiang J (2017) Implications of glycerol metabolism for lipid production. Prog Lipid Res 68:12–25. https://doi.org/10.1016/j.plipres.2017.07.002
Li X, Li W, Zhai J, Wei H (2018) Effect of nitrogen limitation on biochemical composition and photosynthetic performance for fed-batch mixotrophic cultivation of microalga Spirulina platensis. Bioresour Technol 263:555–561. https://doi.org/10.1016/j.biortech.2018.05.046
Acknowledgments
The authors would like to thank CAPES (Coordination for the Improvement of Higher Education Personnel), CNPq (National Council of Technological and Scientific Development), MCTIC (Ministry of Science, Technology, Innovations and Communications) and the Program to Support the Publication of Academic Production/ PROPESP/FURG/2018 for providing financial support that made this research possible.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Etiele Greque de Morais. The first draft of the manuscript was written by Etiele Greque de Morais and all authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Morais, E.G., Nunes, I.L., Druzian, J.I. et al. Increase in biomass productivity and protein content of Spirulina sp. LEB 18 (Arthrospira) cultivated with crude glycerol. Biomass Conv. Bioref. 12, 597–605 (2022). https://doi.org/10.1007/s13399-020-00934-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00934-4