Abstract
Geopolymer concretes have emerged as an alternative to traditional Portland cement concretes with high strength, good durability, well corrosion performance and high-temperature resistance, and being a sustainable and environmentally friendly material. In this study, a comprehensive microstructural analysis of low-calcium fly ash-based geopolymer concrete samples with different alkali activator to binder ratios was conducted after exposure to temperatures ranging from 400 to 800 °C. The experimental results of the geopolymer concrete specimens found out significant findings, including a notable loss of mass and an approximate 80% decrease in compressive strength after exposure to 800 °C. The microstructural analysis underlined crack formation, voids and porosities in the geopolymer matrix at elevated temperatures, affecting the physical and mechanical properties of the material. The study presents significant insights into the behaviour of low-calcium fly ash-based geopolymer concrete with different binder and alkali activator ratios under high temperatures, revealing the performance of geopolymer concretes in extreme environments and the effect of incompatibility between geopolymer concrete and aggregate due to thermal temperature effects on this performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, demand for energy increases as a result of industrialization and technological developments. The usage of fossil fuels like raw slate, coal and natural gas for energy consumption causes increased carbon dioxide (CO2) emissions. On the other hand, the urbanization that emerges due to increasing population on a global scale increases concrete consumption. Traditional Portland cement used in concrete production is obtained by burning limestone. CO2 gas is released into the atmosphere during this process [1, 2]. Therefore, instead of traditional Portland cement for the concrete production as a structural material, reducing CO2 emissions by using an environmentally friendly, sustainable building material is an effective and economical way against global warming [3, 4].
Geopolymer concrete is obtained by alkali activation synthesis of industrial wastes like aluminium and silicium-rich fly ash, blast furnace slag and silica fume [5,6,7]. The geopolymerization mechanism basically consists of the dissolution, transport and polymerization steps of Al and Si particles. Extra dissolved silicates enhance the concentration of dissolved silicium, as the geopolymerization is significantly affected by the Si/Al ratio in the geopolymer gel phase and the amorphous nature of the raw material [8]. An alkaline hydroxide solution with low silica content or alkali hydroxide-alkali silica solution is preferred as activation solution [9, 10]. The most crucial step of the geopolymerization is dissolution step of aluminosilica compounds, and the gel formation depends on the amount of dissolution. Aluminosilicate solids react with an alkaline solution, forming a gel phase on their surface. As the gel phase toughens, the discrete aluminosilicate particles bond together as they diffuse from the particle surface into the gaps between other particles [11]. Geopolymerization process forms amorphous to semi-crystalline aluminosilicate inorganic polymers from an amorphous silicon-containing and aluminium-rich solid and a firmly basic solution [12]. Therefore, the degree of crystallization for geopolymeric products depends on the initial formulations and synthesis conditions such as the degree and duration of the applied heat treatment, and these synthesis conditions that provide crystal formation are very important for determination of product properties. Additionally, geopolymer structures are affected by the water, SiO2 and AlO3 contents of the raw material, likewise the alkali activator source and concentration used for the reaction and the ambient conditions [13].
The geopolymer concrete obtained after the geopolymerization process has some advantages over traditional Portland cement. These properties are listed as high strength, adjustable setting time, low creep and shrinkage behaviour, superior resistance to acid and sulphate attack in addition to thermal conductivity and fire resistance [14,15,16,17,18]. The fire resistance property is crucial for both buildings, structures with a high risk of exposure to high temperatures, accidents and especially industrial structures where dangerous chemicals, nuclear materials, etc., are stored. As known, fire accidents can cause loss of life and property by destroying structures. Repair and strengthening processes in damaged buildings after fire cause economic losses and loss in time and comfort. However, reinforced concrete buildings constructed using traditional concrete are generally fire resistant. However, the concrete binder gel (C–S–H) is destroyed by dehydration at temperatures above 400 °C. Additionally, a hot layer may crack and fracture the concrete because fire creates a significant temperature gradient [19]. The microstructure of the geopolymer gel exposed to heat plays an important role in its fire resistance. Aluminosilicate hydrate (N–A–S–H) with various compounds is the primary binding phases in fly ash-based geopolymer concrete [20, 21]. The gel microstructure determines the structure of the pore network in geopolymer concrete (GPC), and it is observed to control moisture by increasing the folds and pores in the pore network at high temperatures. It is believed that these changes in the pore structure provide resistance to disintegration and fragmentation at elevated temperatures, such as fire, thanks to the water vapour trapped in the structure being released more easily and the internal water vapour pressure decreasing [22, 23]. Hasan et al. [24] informed that the fire resistance of GPC is superior to that of traditional Portland cement concrete, especially at elevated temperatures above 400 °C. Sivasakthi et al. [25] informed that fly ash-based geopolymer mortar, in which copper slag was used as aggregate, performed high thermal stability without any spillage in the temperature ranging from 200 to 1000 °C. All these reasons led researchers to study on the behaviour of GPC at high temperatures. These studies show that GPC performs better fire resistance and thermal stability than OPC, thanks to inorganic frameworks in their chemical structures [14, 26, 27].
Changes in physical and mechanical features with changes in microstructure of fly ash-based geopolymer mortar or paste specimens under heat were investigated in most of the previous studies in the literature [28,29,30,31]. On the other hand, changes in physical, mechanical and microstructural characteristics of GGBS or slag geopolymer mortar specimens mixed with fly ash at varying rates at high temperatures have been reported in some studies [5, 32,33,34,35,36]. Thus, the changes of geopolymer mortar samples produced with different binders after subjected to heat were studied and compared with fly ash-based geopolymer samples. However, fly ash geopolymer bindings that strengthen by polypropylene microfibres with varying mixing ratios were investigated to ensure superior resistance to crack initiation and development at high temperature [37]. In order to detect the microstructural properties and changes at elevated temperature, some analyses such as X-ray diffraction (XRD), scanning electron microscopy (SEM), thermal gravimetric analysis (TGA), XRD and energy-dispersive X-ray (EDX) analysis were used. After geopolymer mortar samples treated to high heat, it was noted that cracks and deteriorations increased with high temperature in all mortar samples by SEM analysis. It was determined that the porosity of the geopolymers, both interior and exterior damages, microstructure compactness and the occurrence of crystalline structures all affect the maximum compressive strength of the geopolymers by using XRD analysis [38,39,40]. Although there are less investigations on the microstructural as well as mechanical characteristics of geopolymer concretes subjected to high temperatures than on geopolymer mortar and paste, there are some studies in the literature. At this point, whether geopolymer samples contain coarse aggregate is important in terms of simulating real-life usage of concrete at elevated temperature. Shaikh and Vimonsatit [41] evaluated the strength of fly ash geopolymer concrete at high temperature in their studies simulating the fire environment and compared it with OPC. XRD and nuclear magnetic resonance methods indicated that the microstructure of GPC remained relatively stable at temperatures below 600 °C. Zhao et al. [42] conducted a study detecting the effects of temperature on the mechanical characteristics of geopolymer concrete, mortar and paste produced using fly ash and blended slag. It was revealed that sulphoaluminate gel with fly ash had higher heat resistance than C–S–H gel with slag by using microstructural studies. In addition, it is revealed that the type of binder in GPC is also effective on the strength at elevated temperatures. According to these studies, another important issue is aggregate size in the geopolymer concrete. It was informed by Zhang et al. that geopolymer with coarse aggregate greater than 12 mm performed higher strength at high temperature with thermal and ambient curing [14]. Kong et al. [7] compared the strength of geopolymer with metakaolin and fly ash at high temperature and showed that geopolymer with fly ash had higher strength. They attributed this to sintering reactions of unreacted fly ash particles. In another study, they reported high-temperature effects on the strength of geopolymer paste, mortar and concrete. Accordingly, they found that decrease in the strength of geopolymer concrete compared to the mortar and paste was the incompatibility between geopolymer concrete and aggregate due to thermal expansion [43].
It is concluded in the literature that geopolymer concrete exhibits high performance such as fire resistance, chemical stability and thermal insulation than OPC concrete at elevated temperatures. In cases where disasters such as fire or exposure to high temperatures may occur, this superiority is very important buildings and other structures. On the other hand, some adverse condition such as loss of strength, microstructural damage and thermal expansion can be observed in GPC at extreme high temperatures. Due to this, changes in the physical and mechanical properties of geopolymers after exposure to high temperature were investigated in terms of colour change, mass loss, compressive and bending strengths, whereas microstructural changes are investigated generally more superficially. However, possible reason for this superiority in mechanical behaviour or strength is believed to be the microstructure of the geopolymer that changes at high temperatures. In addition, most studies focusing on behaviour and microstructural changes at elevated temperatures are conducted on geopolymer mortar and paste, and studies on geopolymer concrete are relatively few. However, it is reported that the distribution and compatibility of geopolymer concrete in the matrix, especially the coarse aggregates, is important at high temperatures [7, 43]. Due to all these reasons, it is important to reveal the stability of geopolymer concrete under thermal effects from a microstructural point of view. Additionally, a study attributed to alkali activator ratios changed by activating low-calcium fly ash-based geopolymer concrete is rarely encountered in the literature. Na2SiO3/NaOH (S/H) varying from 1.5 to 3.5 and alkali activator/fly ash (A/F) ratios varying from 0.5 to 0.7 were also considered in the study. Thus, changes in the microstructure of low-calcium fly ash-based geopolymer concrete specimens with different ratios of alkali activator exposed to 400, 600 and 800 °C were studied. Also, the relationship between these changes with compressive strength and mass loss was reported. Scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) and X-ray diffraction (XRD) analyses were used to detect changes in microstructure. According to the results, changes in intensities of amorphous and crystal content, new crystal formation and densification occurred in the microstructure of specimens treated at high temperatures. The cracks, pores and voids formed in the microstructure, resulted in a decline in the compressive strength of samples at high temperatures.
2 Experimental
Low-calcium fly ash-based geopolymer samples were produced and subjected to elevated temperature in the experimental work. The samples were left at room temperature for cooling using standard air cooling once the temperature reached 400, 600 and 800 °C. Then, the compressive strength was obtained for the GPC specimen exposed to heat, and microstructure analysis was applied to the samples.
2.1 Materials
2.1.1 Fly Ash
Low-calcium Class F fly ash procured from İsken Sugözü thermal power plant, Adana, Turkey, was used for the geopolymer specimens in this study. Low-calcium Class F fly ash conforming to ASTM C 618 has a low Ca content, with 3.98% of the total composition. The FA in the current study is characterized by the amount of SiO2 > 40%, Al2O3 < 30% and CaO < 10%. Thus, it is defined as high content of SiO2 (55.9 wt.%) and Al2O3 (20.7 wt.%) with low CaO content (3.98 wt.%). Almost 85% of fly ash particles in the study were finer than 45 µm. Table 1 shows the chemical component of fly ash identified by X-ray fluorescence analysis.
2.1.2 Alkaline Solution
Sodium hydroxide (NaOH) and sodium silicate (Na2SiO3) were used to make the alkaline solution for the geopolymer binders in this research. 14 M of sodium hydroxide (NaOH) was obtained using 99% pure sodium hydroxide pellets. NaOH solution was given the time to cool down at ambient temperature for 24 h before it was used for production. The solid concentration of Na2SiO3 was 44.05%, with SiO2 at 29.64%, Na2O at 14.41% and H2O at 55.73%. The mass density of sodium silicate was 1510 kg/m3.
2.1.3 Aggregates
The fine aggregate was river sand with diameters of 0.2 to 0.4 mm, and the coarse aggregate was crushed gravel with grain sizes ranging from 7 to 14 mm. The moisture content of the aggregates is achieved with a dry surface that is as saturated as possible to minimize the absorption of water and chemicals.
2.2 Mix Proportions
The compositions of the mixture in the research were based on different amounts of sodium hydroxide and sodium silicate as alkaline activators. Moreover, the GPC mixtures were designed with respect to the different ratios of fly ash to alkaline activators. The ratio of sodium silicate to sodium hydroxide (Na2SiO3/NaOH), denoted as the alkali activator ratio, changed to 1.5 and 3.5, respectively. The rate of liquid alkali activator /fly ash was used as 0.5 and 0.7. The aggregates and fly ash weights were fixed for all mixture compositions. Besides the amounts mentioned, no further water was added to the mix. Mixture amounts of geopolymer specimens are tabulated in Table 2.
The decomposition of silica and alumina during the geopolymerization, which results in an aluminosilicate gel, is crucial for the geopolymer's stability throughout the hardening phase, providing the geopolymer with superior compressive strength. Thus, rates that will influence silica and alumina's dissolution were selected as the study's variables [20, 44].
2.3 Specimen Preparation
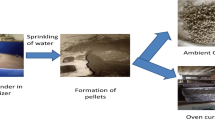
Initially, the fine and coarse aggregates, as well as the fly ash, were fully dry-mixed in the mixer for 2 min. After addition of the Na2SiO3 solution to the dry mixture, the NaOH solution that had been made earlier than 24 h was gradually poured into the mixture for 5 min. The mixture had no additional water or plasticizer at the end of 8 min. In the study, the moulds and geopolymer specimens were both cured for 24 h at 90 °C in a vented furnace. Afterwards, samples were allowed to cool and demoulded at ambient temperature. Before strength tests, specimens that had been taken from the moulds were kept at ambient temperature for 28 days. There is no reinforcement in the GPC specimens produced in the current study. Three specimens of each admixture were produced, and the mean values were calculated at the end of each test.
2.4 Experimental Method
2.4.1 High-Temperature Tests
The high-temperature oven was preferred in the current research in order to investigate the characteristic of geopolymer concrete specimens under high temperatures up to 800 °C. Cube specimens arranged in groups at various temperatures as ambient temperature, 400, 600 and 800 °C, were used for each mix to stimulate fire effects. Following furnace activation, the interior temperature rises gradually at a rate of 10 °C/min. The GPC specimens were kept constant for one hour at each desired temperature [18]. The cube samples were then removed and allowed to cool to ambient temperature before tests. Following exposure to high temperatures, investigations on weight loss, compressive strength and microstructure were conducted (Fig. 1).
2.4.2 Weight Loss
The changes in the weight of subjected GPC specimens were determined before the compressive strength test. The size of the specimen was 150 mm cube. The loss in weight was measured by weighing the samples before and after exposure to heat using Eq. (1).
where W.L. was the weight loss, Wb referred to the specimen’s weight before being subjected to the desired temperature, and Wa denoted the weight of the specimen after being heated.
2.4.3 Compressive Strength Test
The compressive strength test was conducted utilizing a compression test equipment at an axial loading rate equal to 0.5 MPa/s until the concrete specimens were crushed. The average test results of three specimens were considered for strength analysis. The residual strength of each geopolymer specimen after exposure to elevated temperature was measured in this way. It was used to determine the residual strength of each geopolymer concrete specimen after heat.
2.4.4 Microstructural Analysis
After compressive strength tests, the crushed specimens were submerged in acetone to stop the geopolymerization. Then, the specimens were dried in the desiccators under a vacuum. The samples were ground using a pulverizer before being sieved through a 75 micron sieve; the X-ray diffraction (XRD) examination was applied on the powder that was retained on the 75 micron screen [45]. XRD is used to detect crystal phases in various compounds and to analyse these phases quantitatively. The machine used for the XRD was PANalytical Empyrean. Step scanning was carried out on the samples in the range of 2θ = 5°–65° at a rate of 1°(2θ) per minute. Before and after being subjected to heat, GPC specimens of about 2.5–5 mm in size were obtained, sawed, varnished and dried in a desiccator at 60 °C with ethanol for 24 h before SEM and EDX analysis. A Zeiss/GeminiSEM 500 was used to detect the morphology and microscopic features of specimens of exposed and unexposed geopolymer concrete. To identify the chemical composition of the samples, EDX was operated by EDAX software.
3 Results and Discussion
3.1 Weight Loss
Both unexposed to high temperature and exposed specimens were evaluated regarding their weight loss. Before exposure to heat, the GPC cube samples were weighed. One day after removing from the furnace, specimens were weighed again to calculate the weight loss by temperature. In this way, each mix's weight loss for target temperatures was calculated and is given in Table 3. As shown in the table, there is a weight loss with temperature, and as the temperature rises, the total weight loss of the specimen increases. According to the alkaline activator to binder ratio, while it equals 0.7, the overall weight loss of each elevated temperature is the highest value due to the higher water/fly ash ratio. The ratio of sodium silicate to sodium hydroxide increases; the weight loss decreases at 400°C. On the contrary, it is different at 600 and 800°C since the alkaline activator ratio increases and the weight loss increases. One reason is that higher compressive strength leads to higher weight losses, according to the study by Zhao et al. [42]. Also, they revealed that higher strength causes more mass losses above 300 °C due to the mass losses of the GPC specimen up to 200 °C attributed to water evaporation and content playing a role in the mass loss [42].
The most important physical change observed in geopolymer concrete was weight loss. This is attributed to the loss of free water or bounded water. Weight loss is considered due to evaporation of free water. Moreover, the mass loss is mainly due to dehydration of the geopolymer gel and dehydration of hydroxyl groups. Thermal shrinkage was observed in geopolymer as a result of dehydration and weight loss. The changes in weight loss of each temperature concerning the previous weight of the relevant temperature are shown in Fig. 2. Geopolymer specimens of all mixes had a rapid loss in weight up to 400°C due to moisture evaporation in the specimens. The weight losses of the specimens then gradually increased between 400 and 600 °C before increasing once further above this temperature. Although the increase in further temperature, minor weight changes are observed in Fig. 2. Thokchom et al. [30] obtained a similar outcome in their study and decided that the weight loss in samples is related to the moisture evaporation in the samples. The moisture starts to evaporate after 100°C, and the chemically bound water in the structure continues to evaporate up to 300°C. Klima et al. [46] indicated in their review study that the most important temperature interval for weight loss was between 20 and 300°C since 80% of total weight loss observed because of the loss of adsorbed water and water loss in geopolymeric gel. Also, they explained in their review study that a reason for the loss of mass was the significant loss of free and pore water below 250°C, while the reason was the geopolymerization process of free hydroxyl groups such as Si–OH and Al–OH further up to 800°C due to water removal by condensing silanol and aluminol groups in the geopolymer gel.
3.2 Residual compressive strength
The experimental results of compressive strengths of GPC samples before and after exposure to heat are presented in Table 3. Four mixes of geopolymer concrete samples were investigated to evaluate the performance of GPC at high temperatures and assess the effect of alkaline activator and binder ratios under elevated temperatures. As given in Table 3, the peak compressive strength is at ambient temperature for X3.5Y0.7, X3.5Y0.5 and X1.5Y0.7, respectively. After exposure to 400 °C, the compressive strength of X1.5Y0.5 reaches its peak, which is a small increase. On the contrary, the compressive strengths of other mixes decrease with temperature. The variance in the compressive strength of the GPC specimens is related to the various ratios of sodium silicate solution to sodium hydroxide solution and alkali activator to fly ash since the mixing, curing and age of the samples were the same. Accordingly, S/H is equal to 1.5 performed higher decrease than S/H equalling to 3.5. In terms of A/F ratios, higher decrease was 0.7, whereas the lower decrease was 0.5 under elevated temperature. Thus, lower S/H was resulted in higher decrease of compressive strength and higher A/F caused more reduction of compressive strength with elevated temperature, also. A minor rise in compressive strength of X1.5Y0.5 at 400 °C can be explained by the fact that GPC provides higher compressive strength at higher temperatures, as many researchers have found before [35, 47,48,49]. At temperatures between 100 and 300 °C, they observed that particles containing hydroxyl ions (-OH) were bonded together by a dehydration mechanism that released water to form a bigger particle. Then, fly ash-based geopolymer concrete that included Si–OH compounds, bonded hydroxyl group to silicon, was released at elevated temperatures, especially 100 and 300 °C. This structure related to Si–O–(Si or Al) provided increases in connectivity, strength and stability thanks to the densification of geopolymer [50,51,52]. Thus, the rise in compressive strength at 400 °C occurred due to the ongoing reaction in X1.5Y0.5 specimens caused by the sintering reactions of unreacted fly ash at high temperatures, which formed a more dense microstructure [7, 14, 28, 53]. This can also be associated with the OH ions in the X1.5Y0.5 mix, which accelerated dissolution, were higher in the NaOH solution, while the amount of Na2SiO3 was lower. Because of the number of OH ions, it showed rapid chemical reactions in the geopolymerization, which provides higher strength at 400 °C [54, 55]. Between 400 and 800 °C, the residual strength of the lower Na2SiO3 to NaOH ratio was greater than that of any other mix. Similarly, the residual strength of the lower ratio of alkali activator to fly ash was higher than another mix because of the large amount of fly ash. As the temperature increased, the differences in the residual compressive strength between the mixtures according to the alkaline activator ratios and alkali activator to binder ratios decreased.
The variation of residual compressive strength of geopolymer concrete after exposure to elevated temperature is illustrated in Fig. 3. Loss in compressive strength decreased gradually to 400, 600 and 800 °C for the X3.5Y0.7, X3.5Y0.5 and X1.5Y0.7 specimens. From ambient temperatures to 400 °C, the residual strength of X1.5Y0.7 increased slightly to 3.09%, whereas from 400 to 800 °C, the residual strength decreased gradually. The residual strength of this specimen was 61.81% at 600 °C and 22.40% at 800 °C. Residual compressive strength at 400 °C was approximately 83.29% for X3.5Y0.5, 79.70% for X1.5Y0.7 and 63.78% for X3.5Y0.7 specimens. These specimens performed continuous loss of compressive strength with a rise in temperature. Thereafter, the strength results designated that the X3.5Y0.5 mix maintained about 60% of its strength, while the X1.5Y0.7 mix retained about 46% of its strength, and the X3.5Y0.7 mix retained about 40% of their strengths at 600 °C. However, all GPC specimens lost approximately 70–80% of strength at 800 °C. Therefore, a significant loss in compressive strength was recorded after exposure to the highest temperature of 800 °C.
As seen in Fig. 3, the compressive strength losses of all GPC samples at high temperatures might be attributed to water evaporation, thermal discrepancy between the aggregates and the geopolymer mixture, cracks and voids with changes in microstructure. The evaporation of free and combined water or the movement of bonded water and OH groups in all geopolymer specimens lead to loss in strength above 400 °C [6, 53, 56, 57]. After water loss, there is a disparity among the aggregates with expansion and geopolymer mixture with shrinkage. These changes in the temperature lead to microcracks on geopolymer concrete, causing losses in strength [43, 58, 59]. Beyond 600 °C, the crystallinity of the geopolymer matrix at high temperatures was observed. The geopolymer matrix, an amorphous structure started to transform into a crystalline structure causing larger cracks due to the non-uniform recrystallization at higher temperatures [31, 48]. This is further discussed in later sections. From 600 to 800 °C, the compressive strengths of all GPC specimens were severely reduced with the formation of macrocracks on the matrix because of the deterioration of the cellular framework of the geopolymer. Moreover, some porosities were observed in the microstructure of geopolymer concrete by decomposing the C–S–H gel, burning the residual carbon in fly ash and decarbonizing the calcite [58]. These porosities caused the significant loss in strength of all specimen mix exposed to heat. With the rise in temperature, the macrocracks occurred, porosities increased, and some fragments in the GPC matrix were observed under high pressure because of capillary tension [24, 60]. Also, capillary tension leads to void volumes and increases the void sizes, resulting in the lowest strength in the geopolymer structure at 800 °C [61,62,63].
3.3 Microstructural Characterization
Geopolymerization, which is expressed as the process of amorphous aluminosilicates constituting a new form with alkali hydroxide and silicate solution to form a three-dimensional tetrahedral alumina and silica matrix between amorphous and semi-crystalline, is the basis for the chemical reaction mechanisms in the production of geopolymer concrete. The geopolymerization process, which begins with dissolution, continues with the precipitation of an aluminosilicate gel that hardens into a three-dimensional tetrahedral aluminosilicate matrix and finally ends with nucleation and particle growth. Thus, the first step of geopolymerization begins with the dissolution of amorphous silica (aluminosilicate) in solution. Removal of a Si or Al cation leads to the release of other bound cations, which are attacked by more hydroxyl ions. Initial hardening of geopolymers occurs when the dissolution rate is exceeded by the rate of condensation and precipitation of aluminosilicate. However, expulsion of a liquid from the formed gel occurs as the gel network continues to densify, and the expulsion of water initially consumed during dissolution leads to the hardening of the aluminosilicate matrix. As silica dissolves in solution, a simultaneous precipitation reaction occurs with the precipitation of aluminosilicate gel, where the silica layer in crystalline or amorphous form is deposited. Another stage is crystal growth, which generally consists of two different phases called nucleation and particle growth. Nucleation can occur randomly at the phase boundaries of molecules in solution or on particle surfaces, or it can occur spontaneously in solution. Particle growth occurs when enough molecules come together to form a crystal [64]. On the other hand, temperature significantly affects the geopolymerization process and gel formation. Various chemical phenomena occur at elevated temperatures, in the microstructure of geopolymer concrete, such as the dissipation of water adsorbed by the aluminosilicate gel around 200–400 °C, the subsequent formation of anhydrous substances and the crystallization of anhydrous products between 600 and 800 °C. These phenomena occurring in the microstructure are discussed in the following sections.
3.3.1 SEM Micrographs Analysis
The SEM micrographs of the unexposed and exposed geopolymer concrete samples with S/H = 1.5 and 3.5 while A/F = 0.5 and 0.7, which were cured at 90 °C, are depicted in Figs. 4, 5, 6, 7 and 8. SEM is one of the suitable methods for the microstructure of geopolymer concrete samples obtained from a smashed sample of the specimen [65]. According to the literature, alkaline silicoaluminate, which consists of aluminium and silicon tetrahedral arranged to shape a 3-D framework, is the primary reaction product of fly ash-based geopolymer. The 3-D framework, depending on the mix of the content and design considerations, provides the superior strength of the geopolymer concrete [66,67,68]. SEM analysis of unexposed samples with various ratios of S/H is given in Fig. 4. The morphology of the unheated geopolymer specimen was smooth and intense and contained some spherical fly ash particles. As illustrated in Fig. 4a–d, the unexposed GPC samples had lots of these spherical particles defined as unreacted fly ash particles of different sizes. However, the fly ash particles were either partly or completely attacked by alkaline solution. Due to this, there were some partly reacted fly ash particles and completely reacted fly ash particles in the geopolymer matrix (Fig. 4a–d). Also, there were a few microcracks in the GPC specimens, which were cured at 90 °C due to water loss and drying shrinkage. In addition, these microcracks in the geopolymer matrix can be explained as occurring either during the compressive strength test or preparation of the sample for SEM. Some small voids appeared in the GPC matrix during the geopolymerization process.
SEM micrographs of geopolymer concrete with different S/H and A/F ratios, which were exposed to elevated structures, are depicted in Figs. 5, 6, 7 and 8 to present the effects of high temperature on the microstructure. Four different specimen series were investigated regarding alkali activated ratios of 1.5 and 3.5, while the alkali activator to fly ash ratio was 0.5 and 0.7, respectively. In all samples, some unreacted fly ash particles still were embedded in the intense geopolymeric matrix after being exposed to 400 °C. Additionally, the fly ash particles in geopolymer gel were either partly or totally attacked by alkaline solution as due to the continuing geopolymerization process. Therefore, both partly reacted fly ash particles and reacted fly ash particles were observed in the geopolymer matrix in Figs. 5, 6, 7, 8a, b. The binder phases appear to adhere to the surface of some unreacted fly ash particles (Figs. 5, 6, 7, 8a, b). The microcracks caused by the severe drying shrinkage occurred on the geopolymer matrix at elevated temperatures. Additionally, some microcracks appeared on the interfacial transition zones between the matrixes of the GPC specimens, especially with the ratio of A/F 0.7 (Figs. 7, 8.a, b). When mostly fly ash particles were in the binder, several voids were identified throughout the geopolymer concrete. Some of them were heterogeneously dispersed in the GPC gel, while others were detected on the surfaces of partly reacted or unreacted fly ash particles. It is believed that this is a typical microstructural feature observation of fly ash-based geopolymer binders [28].
After exposure to 600 °C, unreacted fly ash particles embedded into the geopolymeric gel were identified by an ongoing geopolymerization process. Also, some new needle-shape products appeared around the partly reacted and unreacted fly ash particles, especially in the GPC samples with S/H = 1.5 and A/F = 0.7 and S/H = 3.5 with A/F = 0.5 at this temperature (Figs. 6, 7c, d) [69, 70]. However, some white needle crystals were detected in SEM micrographs of GPS specimens with A/F = 0.5 and A/F = 0.7 for S/H = 1.5 (Figs. 5d, 7c, d). These crystals are believed to be associated with NaOH or Na2SiO3. Na2CO3 or Na2SiO3 precipitation, which is obtained from unreacted Na in geopolymer reactions with ambient CO2, causes these needle-shaped products [71,72,73].
Several voids and cracks appeared on the geopolymer matrix. Additionally, due to the thermal gradients, some cracks occurred on the geopolymer matrix and the interfacial transition zones between the matrix and aggregates. Because of the increase in temperature, a significant amount of sodium was decomposed, which caused pores and cracks in the geopolymer gel, weakening its strength. Besides pores, voids were also detected because of either capillary effects or dehydration of the geopolymeric gel (Figs. 5, 6, 7, 8c, d). As the exposure temperature increases, more cavities indicating a porous structure and pores in the SEM images are illustrated in Figs. 5, 6, 7, 8.e, f. at 800 °C. It is a high possibility that the decomposition of geopolymer gel and phase transformations leading to the collapse of the geopolymer matrix caused the pores in the matrix in Figs. 5, 7, 8e, f [74].
After exposure to high temperatures, bound water and hydroxyl OH in the geopolymeric gel were decomposed chemically, and it was informed that damage occurred in the specimens was composed of thermo-mechanical and thermo-chemical damage at elevated temperatures [75]. Therefore, the microstructure of the geopolymer concrete became loose and porous, leading to significantly lower compressive strength indicating the serious degradation of GPC specimens [39, 76]. Additionally, micro- and macrocracks were detected in the GPC specimens at 800 °C in Figs. 56, 7 and 8e, f. Because the thermal gradients induced thermal stress [77], some microcracks appeared on the interfacial transition zone between the aggregates and mortar in Figs. 7 and 8e, f, while others were observed on the matrix. Some cracks were expanded through the fractured surfaces on the matrix in Fig. 5f. At this temperature, the number of unreacted fly ash particles and higher bright crystal contents increased significantly by comparing lower elevated temperatures in Figs. 6 and 7f. Indeed, the inflatable trails of the high unreacted silicate contents were observed in the geopolymeric gel at 800 °C (Figs. 6, 7f), which caused the microstructural deterioration of the GPC samples. However, there were fewer impurities in the microstructure of geopolymer concrete specimen. Thus, it provided a smoother texture induced by melting, viscous flow and sintering of unreacted fly ash particles and amorphous aluminosilicate gels at 800 °C in Figs. 5f and 8f [74]. Besides smooth texture, viscous sintering resulted in a denser microstructure, as appeared in the SEM micrographs in Figs. 56, 7, 8e, f [78]. Indeed, several voids occurred in the geopolymer matrix, and the void size increased significantly after being subjected to high temperature.
As seen in the SEM micrographs of the exposed and unexposed geopolymer specimen (Figs. 4, 5, 6, 7, 8), there were some differences in the densification, geopolymerization, number of cracks and voids present and size of them, also cavities and porosities at elevated temperature. As the temperature rised, the presence of cracks and voids increased, while their existing was larger than before. Also, porosities and cavities appeared especially beyond 600 °C. Further ongoing geopolymerization was observed, especially regarding products such as needle-shaped crystals at high temperatures. Because of all differences, the strength of the geopolymer samples at the elevated temperature investigated, except the strength of the mix with S/H = 1.5 with A/F = 0.5 at 400 °C, decreased significantly. Besides the strength of samples, the loss in weights of all specimens increased as exposed to heat.
Compared with the exposed samples according to the value of Na2SO4/NaOH, the microstructures of specimens with the S/H ratio of 3.5 and the alkali activator to fly ash ratio of 0.5 were almost similar to each other, but more geopolymerization products occurred in the matrix of samples with A/F ratio of 0.5 after exposure to 400 °C (Fig 5a, b). This indicates a larger number of reacted fly ash, resulting in higher compressive strength for samples with an S/H ratio of 1.5. However, the difference in compressive strength was pretty low. Similarly, there was no significant difference between the microstructures at the same temperature in samples, and the ratio of activator to fly ash was 0.7. While more white needle shapes were formed due to ongoing geopolymerization in specimens with S/H ratio of 1.5 (Fig 5a, b), it was relatively less at a ratio of 3.5 (Fig. 6a, b). The matrix also had larger microcracks (Fig. 6a, b). Therefore, even with a small difference, the compressive strength of the specimen with a ratio of Na2SO4/NaOH of 1.5 was higher than that of samples with a ratio of 3.5. When the microstructures were investigated at 600 °C, more reacted fly ash and white needle shapes formed in the GPC sample with the ratio of 1.5 in consequences of the geopolymerization (Fig. 5c, d). It was observed that the pores in the matrix of the specimen with a ratio of 1.5 (Fig. 5c, d) reduced the compressive strength. The compressive strength of the sample with A/F ratio of 0.5 and S/H ratio of 1.5, was higher than S/H ratio of 3.5 at this temperature. As the temperature increased, both mixes experienced a significant decline in compressive strength. After being subjected to 800 °C, the microstructure of the specimen with the same ratio of A/F (0.5) was compared to those of specimens with the S/H ratio of 1.5 and with the S/H ratio of 3.5, there were large cracks, voids and cavities in the matrix of the specimens for S/H ratio of 1.5 (Fig. 5e–f). On the other hand, lots of unreacted silica phase presences embedded in the matrix of the specimens with S/H ratio of 3.5 was observed (Fig. 6e, f). However, larger cracks and a porous structure were not present as in samples with a ratio of 1.5 (Fig. 5e, f). A denser microstructure was observed for the specimen with a ratio of 3.5 (Fig. 6e, f). Because of all these reasons, the strength of the specimen with S/H ratio of 3.5 was higher than the ratio of 1.5, albeit with a small difference. Nevertheless, the decline in specimen strength was significantly pronounced at 800 °C for both mixing ratios.
Considering the alkali activator and fly ash ratio, the microstructure of the specimen with S/H ratio of 1.5 and A/F ratio of 0.5 exposed to 400 °C was examined; there were more geopolymer products related to the ongoing geopolymerization process and sintering (Fig. 5a, b). However, relatively smaller cracks were observed in the matrix (Fig. 5a, b). Specimens with an A/F ratio of 0.7 had larger cracks and voids in the matrix (Fig. 7a, b). For this reason, higher strength was obtained for the sample with an alkaline activator to fly ash ratio of 0.5. Beyond to 600 °C, the compressive strength of the GPC specimen with A/F ratio of 0.5 was similarly higher. While the microstructure of the sample (Fig. 5c, d) exhibited minor voids, the microstructure of the sample with an S/H ratio of 0.7 displayed microcracks in the interface transition zone (ITZ) between the aggregate and mortar during the sintering process (Fig. 7c, d). Furthermore, as reported by Kong et al. [43], the strength of the mentioned mixture was inferior to others due to the lack of compatibility between the aggregate and the geopolymer concrete matrix (Fig. 8c, d). Although there were independent cavities in the specimen with both mix ratios, it is noteworthy that the specimen with an A/F ratio of 0.7 exhibited cavities that indicated a porous structure within its matrix (Fig. 7c, d). Despite the sudden decrease in strength with increasing temperature, it is important to highlight that the samples with an alkali activator to fly ash ratio of 0.5 performed higher compressive strength. For the samples with a sodium silicate to sodium hydroxide ratio of 3.5, it was observed that existing cracks on the ITZ between the aggregate/mortar expanded through the microstructure of the specimen with A/F ratio of 0.7 at 400 °C (Fig. 8a, b). Due to this reason, the compressive strength of the specimen with a different alkali activator to fly ash ratio was lower compared to the specimen with an A/F ratio of 0.5. Although pore formation started to observe in the sample with a ratio of 0.5 (Fig. 6c, d), the cracks detected in the ITZ of the sample with a ratio of 0.7 expanded and spread into the geopolymer matrix (Fig. 8a, b). In addition, cracks induced by the inconsistency between the matrix and the aggregate became significant after being subjected to 600 °C (Fig. 8c, d). As a consequence of these factors, the compressive strength of this particular sample was reduced at 600 °C. As heated to 800 °C, the specimen with a A/F ratio of 0.5 had a porous microstructure (Figs. 5, 6e, f). On the other hand, the specimen with a ratio of 0.7 displayed numerous significant voids and cavities that emerged within the geopolymer matrix (Figs. 7, 8e, f). These induced a further decline in the compressive strength of this sample, leading to a smaller strength value for the corresponding mixing ratio.
Microstructure and strength changes were investigated at high temperatures, and also the differences in microstructures of samples with different ratios in terms of S/H and A/F at elevated temperatures were evaluated. When the microstructures of the specimens with S/H ratio of 1.5 were investigated generally, lower densification, microcracks and the presence of many independent voids were observed. Larger voids, cavities and significant microcracks in the interface transition zone of the specimens with A/F ratio of 0.7 were detected. These microcracks play a significant role in the reduction of compressive strength, and this phenomenon is commonly referred to as thermal incompatibility between concrete components [79]. Although there was a small difference, the strengths of the mixtures with these ratios were lower due to all these. By considering the final temperature to which the geopolymer concrete specimens were exposed, the specimen with an S/H ratio of 3.5 and A/F ratio of 0.5 exhibits superior compressive strength compared to mixtures with other ratios. With an increase in the sodium hydroxide content in the activator, there is a corresponding increase in the presence of unreacted or partially reacted fly ash within the geopolymer matrix. Thus, the rise in the activator content adversely affects the compressive strength at high temperatures [79]. In addition, mixtures containing a high proportion of activators form aluminosilicate gel with a high reaction density but also contain a high amount of unreacted silicate products. Therefore, it reduces the strength at elevated temperature. Conversely, a high Si/Al ratio is also believed to cause densification and sintering processes. Both the amounts of Na2SO4 and fly ash are high, which indicates a high amount of silicon and the strengths of samples at high temperatures increase. In addition, mixtures containing high proportions of activators form an aluminosilicate gel with a high reaction density but also contain a high amount of unreacted silicate products.
3.3.2 EDX Analysis
Energy-dispersive X-ray (EDX) analysis with SEM was used to investigate the microstructure of fly ash alkali-activated geopolymer concrete cured at 90 °C both before and after exposure to the high temperature. EDX spectra were obtained from various locations labelled as A, B, C, D, E, F, G and H on SEM micrographs of unexposed and exposed specimens at temperatures of 400 °C, 600 °C and 800 °C. Based on the provided EDX graphs in Figs. 9, 10, 11 and 12, it is evident that the constituent elements including silicon (Si), oxygen (O), aluminium (Al), sodium (Na) and iron (Fe) are present in both the exposed and unexposed specimens. Although in trace amounts, the EDX spectra of all geopolymer specimens also revealed the presence of magnesium (Mg), potassium (K) and calcium (Ca) phases. However, carbon (C) element was identified in the EDX spectra of the specimens with S/H ratios of 1.5 and 3.5 at ambient temperature and the ratio of 3.5 at 600 °C. It should be noted that the changes in the constituent elements may not provide any clear indication because of the different selected spots for comparison. Geopolymer gels or unreacted particles and fly ash particles constituted the final products' microstructure. The formation of sodium aluminosilicate hydrate (N–A–S–H) gel occurs by employing sodium hydroxide as the alkaline activator, sodium silicate as the alkaline binder and low-calcium fly ash as the binder. The matrix exhibited a spongy appearance with a smooth surface, closely resembling a gel structure characteristic of N–A–S–H type [80]. The existence of elements such as Si, O, Al and Na in the EDS spectra of the fly ash-based geopolymer signifies the existence of N–A–S–H gel.
As seen in Table 4, the amount of Si was high in all specimens because of presence in fly ash and matrix. Na content mainly diffused in the GPC matrix was relatively high in the samples, and the presence of it was derived from the alkaline activator solution. Among the elements contributing to the formation of N–A–S–H gel, aluminium (Al) exhibited a significantly high concentration following silicon (Si) and sodium (Na) in all geopolymer samples [80]. However, Ca distributed in the fly ash particles was relatively less in the GPC matrix. The intensities of Mg and Fe were pretty low in the matrix. K was distributed in the matrix with a small amount, which was negligible as compared with other elements. A relatively high amount of O element was detected in all samples due to fly ash particles consisting of a high SiO2, and the O element was almost uniformly distributed in the geopolymer matrix. Indeed, the C element was detected in X1.5Y0.5 and X3.5Y0.5 mixtures at ambient temperature and 600 °C with a higher amount than other elements except O and Si. It is believed that the formation of Na2CO3 from unreacted Na in geopolymer reactions with CO2, which indicates the white needle crystals at ambient temperature for both mixtures and at 600∘C for the X3.5Y0.5 mix, may be the reason for the existence of C in the samples. Since this microstructure corresponded to the strength improvement of the specimens, the strength of specimens, where the C element was identified, was higher than the specimens at the same temperature [72].
In Fig. 13, the relationship between the atomic percentage of main elements, compressive strength and temperature was investigated. With the exception of silicon (Si), the atomic percentages of the main elements in the X1.5Y0.5 sample showed variability as the temperature increased. The atomic % of Si rose by temperature, while the compressive strength decreased at elevated temperatures, except 400 °C. At 400 °C, atomic % of Si, Al and Ca increased, and also compressive strength increased even with a small amount. It indicated that the geopolymerization process, which was associated with forming of ongoing N–A–S–H gel, continued to develop at this temperature. In contrast, Na% decreased at the same temperature [81]. Although the atomic % of Si and Ca rose, the atomic % of Na and Al dropped after exposure to 600 °C. New crystallization appeared by increasing the Si and decreasing Al, while some unreacted fly ash embedded in the matrix occurred. It led to a gradual degradation in compressive strength of the specimen at 600 °C. As the temperature increased, the compressive strength consistently declined. After specimens exposed to 800 °C, atomic % of Na, Al and Si increased, whereas atomic % of Ca decreased. The densification and new crystal formation in the matrix as well as the full destruction of N–A–S–H gel owing to melting and crystallization occurred at this temperature due to changes in the microstructure, which decreased the compressive strength of the X1.5Y0.5 specimen [36].
According to the relationship in Fig. 14, the atomic % of the main elements fluctuated with an increase in temperature for the X3.5Y0.5 mix. After exposure to 400 °C, the atomic % of all main elements as Si, Na, Al and Ca increased due to the new crystallization phase of Na-Al-Si phases in geopolymer with fly ash [81]. Then, the strength of the sample gradually declined. In contrast, the atomic % of all main elements decreased at 600 °C when the temperature increased. This is likely because the crystallization is alternates to new phases, such as anorthoclase and nepheline. As a result, the mineral phases may decrease in density, which indicates a decrease in the number of main elements. Besides this, the compressive strength of the samples subjected to 600 °C decreased again. The decrease in the strength of Si–O–Na bonds is considered indicative of the weakening of polymer cross-linking chains and overall degradation of properties [81]. By increasing in temperature from 600 to 800 °C, the atomic % of Si, Na, Al and Ca increased, which resulted in the sintering of the fly ash particles and additional geopolymerization process. Additionally, densification in the sample matrix was occurred, while the compressive strength continued to decrease at this elevated temperature.
Table 5 presents the atomic ratios of the elements included in the geopolymer samples at ambient and elevated temperatures. These ratios were calculated using the data obtained from the EDX spectra of spots A–H shown in Figs. 9, 10, 11 and 12. Locations A–B, C–D, E–F and G–H correspond to the different temperature conditions of the geopolymer samples, specifically ambient temperature, 400 °C, 600 °C and 800 °C, respectively. The atomic ratios of Si/Al, Ca/Si, Mg/Si, Na/Si and Al/Ca are given in Table 5. The samples with various alkaline activator ratios and alkaline activator to binder ratios had higher Si/ Al and Al/Ca ratios, while Mg/Si ratio was very low in all specimens. The atomic ratios of Si/Al, Ca/Si, Na/Si and Al/Ca in the X1.5Y0.5 mix at ambient temperature were within the limited ranges of 2.89, 0.13, 0.61 and 2.72, respectively. After exposure to 400 °C, the atomic ratios of Si/Al and Na/Si decreased, whereas the atomic ratios of Ca/Si and Al/Ca increased. Then, Si/Al and Ca/Si ratios increased, whereas Na/Si and Al/Ca decreased at 600 °C. As the temperature increased at 800 °C, the atomic ratios of Si/Al and Al/Ca increased, while Na/Si and Ca/Si decreased. In the X3.5Y0.5 mix, the atomic ratios of Si/Al, Ca/Si, Na/Si and Al/Ca at ambient temperature were within the limited ranges of 2.65, 0.13, 0.50 and 2.86, respectively. Beyond 400 °C, the atomic ratios of Si/Al and Al/Ca increased, whereas Na/Si and Ca/Si decreased. As exposed to 600 °C, the atomic ratios of Si/Al increased, while Na/Si and Al/Ca atomic ratios decreased, and the ratio of Ca/Si remained the same at 600 °C. The atomic ratios of Si/Al and Al/Ca decreased, whereas Ca/Si decreased, and Na/Si remained the same at 800 °C.
As seen in Table 5, the ratios of Si/Al, Na/Si and Al/Ca are variable with the temperature. Also, other ratios of Ca/Si and Mg/Si are variable, but this difference was quite low. Besides this, the ratios of Si/Al, Na/Si and Al/Ca were considered while evaluating the EDS spectra analysis in the literature [36, 82]. According to the ratios in Table 5, The variations in the quantities of silicon (Si), sodium (Na) and aluminium (Al) play a crucial role in the geopolymer matrix and its overall process, particularly at elevated temperatures, as these elements are essential components of the N–A–S–H gel. Additionally, these ratio changes indicate ongoing geopolymerization process and new crystal formation leading to either an increase or decrease in strength. After heating to 800 °C, the high value of Si/Al in the geopolymer gel resulted in densification and sintering processes in the geopolymer concrete [79]. Moreover, a substantial quantity of excess silicon present in the solution does not undergo reaction with the geopolymeric gel. This remaining unreacted silicate has a tendency to expand when subjected to elevated temperatures, ultimately leading to a decline in strength due to high-temperature exposure [46].
The ratios of Na/Si in X1.5Y0.5 were higher than in X3.5Y0.5 at both room and high temperatures. When these ratios were evaluated with the strength of samples at both room and high temperatures, the strengths of X3.5Y0.5 samples were higher than X1.5Y0.5 except at 400 °C. Furthermore, the optimum Na/Si ratio was reached in X3.5Y0.5 samples at 600 °C, and no ratio was changed at 800 °C. Besides this, GPC samples included in Na-based activator deteriorated rapidly at high temperatures attributed to the crystallization of Na-feldspars and an increment in pore size [46]. Therefore, it is believed that higher Na/Si ratios adversely affect both microstructure and strength of samples at elevated temperatures. Indeed, the excess activator content, which is also related to the amount of Na element, produced a highly unreacted or partially reacted silicate phase and this phase causes a decline in residual strength of the GPC sample beyond 600 °C [79].
3.3.3 XRD Analysis
X-ray diffraction (XRD) analysis was applied to detect the phase composition of the geopolymers, and the XRD results of exposed and unexposed geopolymer concrete samples, which were kept at room and high temperatures, are illustrated in Fig. 15. Peaks in the XRD graphs of the specimens represent crystalline characteristic, whereas the non-peaks represent the amorphous characteristic of geopolymer concrete. The patterns of the unexposed specimens performed the broad hump indicating the amorphous materials in geopolymers. All peaks were positioned between 20° and 85° as 2θ in the XRD pattern of geopolymers, and most of the highest peaks of the samples appeared between 20° and 50° in the XRD graph.
After the alkaline activation process, the XRD analysis of the geopolymer concrete revealed the presence of three primary phases: calcite, quartz and mullite (Fig. 15). The initial phase of the XRD was mullite (M) for all specimens at both ambient and elevated temperatures. In the XRD patterns of all geopolymer concrete samples, the peaks with the highest intensities correspond to quartz, mullite and calcite which are located at specific positions of 2θ. At ambient conditions, mullite exhibits a stable crystalline structure and demonstrates excellent strength and stability. Moreover, it has a minimal thermal expansion even at elevated temperatures [83]. The barrier concentration for crack propagation in quartz, which has a tetrahedral configuration between silicon atoms and oxygen in the hexagonal crystal framework, favourably influences the mechanical properties of geopolymers [33]. Hematite, characterized by the combination of iron and oxygen, exhibits a crystalline structure that belongs to the rhombohedral system. Hematite, which is a non-ferromagnetic phase, contributes to mechanical properties positively such as strength of geopolymer concrete with the stabilisation of the gel, while it decreases the magnetization of geopolymer exposed to elevated temperature [84]. Corundum, one of the primary aluminium oxides, possesses a crystalline structure in the rhombohedral system. Its significant contribution to geopolymers lies in its exceptional hardness, which is comparable to that of diamond [33]. Maghemite belongs to the group of iron oxides and exhibits a cubic spinel crystal structure [33]. It does not participate significantly in the geopolymerization reaction and is susceptible to acid attack due to the pores formed after dissolution. Therefore, unreacted fly ash particles in the matrix are susceptible to both alkali and acid attack. Crystalline compounds are generally more resistant to chemical attack than their amorphous compounds [85]. On the other hand, zeolite is characterized by an Al–O–Si network that encompasses inter-structural Na+ cations formed during the geopolymerization process [33]. The high amount of crystalline zeolite phase in the geopolymer matrix prevents the high mechanical strength [86]. Nepheline crystals, which are rock-forming minerals in the feldspathoid group, belong to the hexagonal system. Crystalline nepheline appeared in solutions of mullite above 600 °C, and it affected the compactness due to the reduction of the total porosity and pore size as newly formed porous crystalline phases [87]. Albite crystallizing in triclinic pinacoidal forms is a member of the Feldspat family. It appeared from the dissolution of zeolite and the reflection of quartz phases above 600 °C and around 800 °C. Since both nepheline and albite are porous and loose microstructure phases, which are attributed to the compactness of the matrix, they adversely affect the mechanical properties such as compressive strength and bond strength of geopolymer concrete [88]. The presence of feldspars, quartz and calcite indicates the pozzolan raw material. The source of calcite in the XRD graph is associated with the raw material, such as aggregate or sand in geopolymer concrete. The calcite positively affects the mechanical properties of geopolymer concrete, while the decomposition of it around 800 °C leads to a reduction in the strength of the geopolymer [89].
The alkaline activation of aluminosilicates led to the formation of the primary reaction product known as the aluminosilicate hydrate gel N–A–S–H. XRD analysis confirmed the presence of N–A–S–H as the predominant product resulting from the polymerization reaction in the samples [90]. Stable amorphous N–A–S–H gels occurred during the polymerization process, whereas crystalline or semicrystalline phases appeared with zeolite. Not only hardened gel composed of Na2O–Al2O3–SiO2 (N–A–S–H) bonds was produced, but also minor quantities of CaO, MgO and FeO2 were formed in the samples after alkaline reaction processes of fly ash with an alkali activator. The alkaline activation of the oxides not only resulted in the generation of a silica gel-like aqueous solution but also facilitated the formation of a hardened geopolymer concrete exhibiting robust bond. On the other hand, NaOH in an alkaline solution helped to enhance the amorphous bonds of N–A–S–H phases [90, 91]. This process provides to obtain superior mechanical and chemical properties by increasing the strength and durability of geopolymer concrete. Also, the semicrystalline phase of N–A–S–H gel appeared in all GPC samples before and after being subjected to 400 °C. All geopolymer concrete samples at ambient temperature showed an amorphous alumino-silicate phase and the crystalline phases of fly ash, such as quartz and mullite. After heating, the intensity of the amorphous broad hump reduced, which indicated decomposition of the geopolymer matrix. Additionally, an increase in temperature caused to form of new crystalline peaks in geopolymer concrete samples. Crystalline phases of the geopolymer concrete in Fig. 15 were almost the same for all specimens at ambient temperature. Crystalline structures observed in the GPC samples were similar to those before the heat exposure up to 600 °C. However, the high temperature altered the amount and strength of crystalline and amorphous phases. It is informed that the thermal stability of zeolite decreased by rising temperature because of the water evaporation and the substitution of Ca by Na, leading to a reduction in strength at elevated temperature [6, 92]. After being heated to 400°C, there was no notable alteration in the XRD pattern compared with those at ambient temperature, with small changes in the intensities of some peaks due to the deterioration of geopolymer gel slightly. With the temperature rising to 600 °C, the intensities of the quartz and mullite peaks exhibited an increase in comparison with the specimens prior to heat exposure (Fig. 15). The observed intensification of the quartz and mullite peaks indicates the decomposition of the aluminosilicate gel. The decomposition resulted in the formation of porosity, leading to a reduction in the compressive strength of the samples at elevated temperatures [35]. On the other hand, zeolite underwent recrystallization into secondary mineral phases as nepheline (NaAlSiO4) and albite (NaAlSiO8) after heat to 600 and 800 °C [83, 93]. In addition, some amount of crystalline nepheline content was observed to form with the major reflections of quartz after sintering at 800°C. Besides this, albite content was also present with Na+ cations after heating during the geopolymerization process. Therefore, the intensities of peaks that indicated the declining amount of the mineral phases, such as quartz and mullite, reduced [35, 94]. As seen in Fig. 15, the XRD patterns of the samples show a drastic change in the intensities of peaks of phases. The intensities of crystalline content were higher, and new crystalline phases were observed after exposure to 800 °C. The phase transformation at this temperature led to changes in the microstructure of the geopolymer matrix. Then, it caused the deterioration of geopolymer concrete by increasing voids and cracks due to thermo-chemical damage. As a consequence of this mechanism, the GPC samples experienced a decline in their mechanical properties [48, 95]. Therefore, it is hypothesized that the formation of the nepheline phase is responsible for thermo-chemical damage, resulting in the degradation of the geopolymer gel matrix and subsequent loss of strength in the geopolymer concrete sample.
Compared to specimens with different alkaline activator and alkaline activator to fly ash ratios, the sample with an S/H ratio of 1.5 and an A/F ratio of 0.5 exhibited a slight increase in compressive strength at 400 °C. This can be attributed to the augmentation of the amorphous phase, potentially leading to further polymerization of initially unreacted components. However, loss of strength was observed in all specimens beyond 600 °C. Higher amorphous phase content, indicating the presence of N–A–S–H gel, promotes increased geopolymerization compared to specimens with lower levels of fly ash and alkaline activators at high temperatures. As explained before, the strength of the specimen at high temperature, especially beyond 600 °C, is reduced compared to those at room temperature due to deterioration of the geopolymer matrix and large cracks, voids and porosity attributed to complex crystallization. In comparing the strength of specimens beyond 600 °C, an increase in fly ash content and sodium silicate resulted in slightly higher strength. This can be attributed to the consumption of sodium silicate, sodium hydroxide and fly ash during the alkaline activation process of fly ash, facilitating the polymerization process at elevated temperatures [82].
4 Conclusion
The study investigated the microstructural changes in low-calcium fly ash-based geopolymer concrete exposed to temperatures ranging from 400 to 800 °C. The weight loss increased while the compressive strength of the specimens decreased at elevated temperatures. No significant differences were observed in terms of compressive strength and weight loss under high temperatures around 800 °C in samples with different alkaline activator and alkaline activator/fly ash ratios. The results demonstrated a significant loss of mass and an approximate 80% decrease in compressive strength at 800 °C. Microstructural analysis found out the crack formation, void, and porosities affecting the physical and mechanical properties of geopolymer concrete. Thermal incompatibility between the aggregates and geopolymer matrix reduced the compressive strength of geopolymer concrete. Therefore, it is crucial to investigate how to stabilize crack formation, improve thermal properties and maintain the residual strength of geopolymer concretes subjected to high temperatures in further studies. Research on various applications such as carbon or steel fibres, which can provide volumetric and strength stability at elevated temperatures and improve the thermal interaction between aggregate and geopolymer concrete interfaces, or additional cementitious materials such as silica fume, metakaolin and wollastonite will be beneficial, especially for the use of geopolymer concretes that can be exposed to elevated temperatures. Also, post-treatment methods such as heat curing or surface coatings can be investigated to increase GPC's resistance to high temperatures and minimize detrimental effects on its properties.
References
Dyson, R.G.: Strategic development and SWOT analysis at the University of Warwick. Eur. J. Oper. Res. 152, 631–640 (2004). https://doi.org/10.1016/S0377-2217(03)00062-6
Huynh, A.T.; Nguyen, Q.D.; Xuan, Q.L.; Magee, B.; Chung, T.; Tran, K.T.; Nguyen, K.T.: A machine learning-assisted numerical predictor for compressive strength of geopolymer concrete based on experimental data and sensitivity analysis. Appl. Sci. 10, 1–16 (2020). https://doi.org/10.3390/app10217726
Davidotis, J.: Properties of geopolymer cements. In: First Int. Conf. Alkaline Cem. Concr. 131–149 (1994)
Hardjito, D.; Rangan, B.V.: Development and properties of low-calcium fly ash-based geopolymer concrete. Aust. J. Struct. 6, 77–86 (2005)
Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S.: The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 47, 409–418 (2013). https://doi.org/10.1016/J.CONBUILDMAT.2013.05.069
Qu, F.; Li, W.; Tao, Z.; Castel, A.; Wang, K.: High temperature resistance of fly ash/GGBFS-based geopolymer mortar with load-induced damage. Mater. Struct. Constr. 53, 1–21 (2020). https://doi.org/10.1617/s11527-020-01544-2
Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K.: Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 37, 1583–1589 (2007). https://doi.org/10.1016/J.CEMCONRES.2007.08.021
Fernández Pereira, C.; Luna, Y.; Querol, X.; Antenucci, D.; Vale, J.: Waste stabilization/solidification of an electric arc furnace dust using fly ash-based geopolymers. Fuel 88, 1185–1193 (2009). https://doi.org/10.1016/J.FUEL.2008.01.021
Khale, D.; Chaudhary, R.: Mechanism of geopolymerization and factors influencing its development: a review. J. Mater. Sci. 42, 729–746 (2007). https://doi.org/10.1007/S10853-006-0401-4/FIGURES/10
Weng, L.; Sagoe-Crentsil, A.K.: Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: part I-Low Si /Al ratio systems. J. Mater. Sci. (2007). https://doi.org/10.1007/s10853-006-0820-2
Xu, H.; Van Deventer, J.S.J.: The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 59, 247–266 (2000). https://doi.org/10.1016/S0301-7516(99)00074-5
Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J.: Geopolymer technology: the current state of the art. J. Mater. Sci. 42, 2917–2933 (2007). https://doi.org/10.1007/S10853-006-0637-Z
De Silva, P.; Sagoe-Crenstil, K.: Medium-term phase stability of Na2O-Al2O3-SiO2-H2O geopolymer systems. Cem. Concr. Res. 38, 870–876 (2008). https://doi.org/10.1016/J.CEMCONRES.2007.10.003
Zhang, H.Y.; Kodur, V.; Wu, B.; Cao, L.; Qi, S.L.: Comparative thermal and mechanical performance of geopolymers derived from metakaolin and fly ash. J. Mater. Civ. Eng. 28, 04015092 (2015). https://doi.org/10.1061/(ASCE)MT.1943-5533.0001359
Khandelwal, M.; Ranjith, P.G.; Pan, Z.; Sanjayan, J.G.: Effect of strain rate on strength properties of low-calcium fly-ash-based geopolymer mortar under dry condition. Arab. J. Geosci. (2012). https://doi.org/10.1007/s12517-011-0507-0
Assi, L.N.; Deaver, E.; Elbatanouny, M.K.; Ziehl, P.: Investigation of early compressive strength of fly ash-based geopolymer concrete. Constr. Build. Mater. 112, 807–815 (2016). https://doi.org/10.1016/J.CONBUILDMAT.2016.03.008
Nath, P.; Sarker, P.K.: Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 66, 163–171 (2014). https://doi.org/10.1016/J.CONBUILDMAT.2014.05.080
Bakharev, T.: Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 35, 658–670 (2005). https://doi.org/10.1016/J.CEMCONRES.2004.06.005
Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K.: Geopolymer concrete: a review of some recent developments. Constr. Build. Mater. 85, 78–90 (2015). https://doi.org/10.1016/J.CONBUILDMAT.2015.03.036
Li, Z.; Liu, S.: Influence of slag as additive on compressive strength of fly ash-based geopolymer. J. Mater. Civ. Eng. 19, 470–474 (2007). https://doi.org/10.1061/(ASCE)0899-1561(2007)19:6(470)
Tang, Z.; Li, W.; Tam, V.W.Y.; Luo, Z.: Investigation on dynamic mechanical properties of fly ash/slag-based geopolymeric recycled aggregate concrete. Compos. Part B Eng. (2020). https://doi.org/10.1016/J.COMPOSITESB.2020.107776
Pilehvar, S.; Cao, V.D.; Szczotok, A.M.; Carmona, M.; Valentini, L.; Lanzón, M.; Pamies, R.; Kjøniksen, A.L.: Physical and mechanical properties of fly ash and slag geopolymer concrete containing different types of micro-encapsulated phase change materials. Constr. Build. Mater. 173, 28–39 (2018). https://doi.org/10.1016/J.CONBUILDMAT.2018.04.016
Hu, Y.; Tang, Z.; Li, W.; Li, Y.; Tam, V.W.Y.: Physical-mechanical properties of fly ash/GGBFS geopolymer composites with recycled aggregates. Constr. Build. Mater. 226, 139–151 (2019). https://doi.org/10.1016/J.CONBUILDMAT.2019.07.211
Hassan, A.; Arif, M.; Shariq, M.: Use of geopolymer concrete for a cleaner and sustainable environment—a review of mechanical properties and microstructure. J. Clean. Prod. (2019). https://doi.org/10.1016/J.JCLEPRO.2019.03.051
Sivasakthi, M.; Jeyalakshmi, R.; Rajamane, N.P.: Fly ash geopolymer mortar: ımpact of the substitution of river sand by copper slag as a fine aggregate on its thermal resistance properties. J. Clean. Prod. (2021). https://doi.org/10.1016/J.JCLEPRO.2020.123766
Mithun, B.M.; Narasimhan, M.C.: Performance of alkali activated slag concrete mixes incorporating copper slag as fine aggregate. J. Clean. Prod. 112, 837–844 (2016). https://doi.org/10.1016/J.JCLEPRO.2015.06.026
Najm, O.; El-Hassan, H.; El-Dieb, A.: Ladle slag characteristics and use in mortar and concrete: a comprehensive review. J. Clean. Prod. (2021). https://doi.org/10.1016/J.JCLEPRO.2020.125584
Pan, Z.; Tao, Z.; Cao, Y.F.; Wuhrer, R.; Murphy, T.: Compressive strength and microstructure of alkali-activated fly ash/slag binders at high temperature. Cem. Concr. Compos. 86, 9–18 (2018). https://doi.org/10.1016/J.CEMCONCOMP.2017.09.011
Fernández-Jiménez, A.; Palomo, A.; Pastor, J.Y.; Martín, A.: New cementitious materials based on alkali-activated fly ash: performance at high temperatures. J. Am. Ceram. Soc. 91, 3308–3314 (2008). https://doi.org/10.1111/J.1551-2916.2008.02625.X
Thokchom, S.; Kr Mandal, K.; Ghosh, S.; Thokchom, S.; Kr Mandal, K.; Ghosh, S.: Effect of Si/Al ratio on performance of fly ash geopolymers at elevated temperature. Springer. 37, 977–989 (2012). https://doi.org/10.1007/s13369-012-0230-5
Payakaniti, P.; Chuewangkam, N.; Yensano, R.; Pinitsoontorn, S.; Chindaprasirt, P.: Changes in compressive strength, microstructure and magnetic properties of a high-calcium fly ash geopolymer subjected to high temperatures. Constr. Build. Mater. (2020). https://doi.org/10.1016/J.CONBUILDMAT.2020.120650
Dudek, M.; Sitarz, M.: Analysis of changes in the microstructure of geopolymer mortar after exposure to high temperatures. Mater. 13, 4263 (2020). https://doi.org/10.3390/MA13194263
Nergis, D.D.B.; Abdullah, M.M.A.B.; Sandu, A.V.; Vizureanu, P.: XRD and TG-DTA study of new alkali activated materials based on fly ash with sand and glass powder. Mater. 13, 343 (2020). https://doi.org/10.3390/MA13020343
Poornima, N.; Sivasakthi, M.; Jeyalakshmi, R.: Microstructure investigation of the Na/Ca aluminosilicate hydrate gels and its thermal compatibility in fly ash–GGBS cementitious binder. J. Build. Eng. 50, 104168 (2022). https://doi.org/10.1016/j.jobe.2022.104168
Kuri, J.C.; Majhi, S.; Sarker, P.K.; Mukherjee, A.: Microstructural and non-destructive investigation of the effect of high temperature exposure on ground ferronickel slag blended fly ash geopolymer mortars. J. Build. Eng. 43, 103099 (2021). https://doi.org/10.1016/j.jobe.2021.103099
Türker, H.T.; Balçikanli, M.; Durmuş, I.H.; Özbay, E.; Erdemir, M.: Microstructural alteration of alkali activated slag mortars depend on exposed high temperature level. Constr. Build. Mater. 104, 169–180 (2016). https://doi.org/10.1016/J.CONBUILDMAT.2015.12.070
Irshidat, M.R.; Al-Nuaimi, N.; Rabie, M.: Thermal behavior and post-heating fracture characteristics of polypropylene microfiber-reinforced geopolymer binders. Constr. Build. Mater. (2022). https://doi.org/10.1016/J.CONBUILDMAT.2022.127310
Çelikten, S.; Sarıdemir, M.; Özgür Deneme, İ: Mechanical and microstructural properties of alkali-activated slag and slag + fly ash mortars exposed to high temperature. Constr. Build. Mater. 217, 50–61 (2019). https://doi.org/10.1016/J.CONBUILDMAT.2019.05.055
Chithambaram, S.J.; Kumar, S.; Prasad, M.M.: Thermo-mechanical characteristics of geopolymer mortar. Constr. Build. Mater. 213, 100–108 (2019). https://doi.org/10.1016/j.conbuildmat.2019.04.051
Hui-Teng, N.; Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Pakawanit, P.; Bayuaji, R.; Yong-Sing, N.; Zulkifly, K.B.; Wan-En, O.; Yong-Jie, H.; Shee-Ween, O.: Comparison of thermal performance between fly ash geopolymer and fly ash-ladle furnace slag geopolymer. J. Non Cryst. Solids 585, 121527 (2022). https://doi.org/10.1016/j.jnoncrysol.2022.121527
Shaikh, F.U.A.; Vimonsatit, V.: Compressive strength of fly-ash-based geopolymer concrete at elevated temperatures. Fire Mater. 39, 174–188 (2015). https://doi.org/10.1002/FAM.2240
Zhao, J.; Wang, K.; Wang, S.; Wang, Z.; Yang, Z.; Shumuye, E.D.; Gong, X.: Effect of elevated temperature on mechanical properties of high-volume fly ash-based geopolymer concrete, mortar and paste cured at room temperature. Polymers (Basel). (2021). https://doi.org/10.3390/polym13091473
Kong, D.L.Y.; Sanjayan, J.G.: Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 40, 334–339 (2010). https://doi.org/10.1016/J.CEMCONRES.2009.10.017
Çelik, A.İ; Özbayrak, A.; Şener, A.; Acar, M.C.: Effect of activators in different ratios on compressive strength of geopolymer concrete. Can. J. Civ. Eng. (2022). https://doi.org/10.1139/cjce-2021-0529
Rashad, A.M.: Influence of different additives on the properties of sodium sulfate activated slag. Constr. Build. Mater. 79, 379–389 (2015). https://doi.org/10.1016/J.CONBUILDMAT.2015.01.022
Klima, K.M.; Schollbach, K.; Brouwers, H.J.H.; Yu, Q.: Thermal and fire resistance of class F fly ash based geopolymers: a review. Constr. Build. Mater. (2022). https://doi.org/10.1016/J.CONBUILDMAT.2022.126529
Khan, M.N.N.; Sarker, P.K.: Effect of waste glass fine aggregate on the strength, durability and high temperature resistance of alkali-activated fly ash and GGBFS blended mortar. Constr. Build. Mater. 263, 120177 (2020). https://doi.org/10.1016/J.CONBUILDMAT.2020.120177
Yang, Z.; Mocadlo, R.; Zhao, M.; Sisson, R.D.; Tao, M.; Liang, J.: Preparation of a geopolymer from red mud slurry and class F fly ash and its behavior at elevated temperatures. Constr. Build. Mater. 221, 308–317 (2019). https://doi.org/10.1016/J.CONBUILDMAT.2019.06.034
Aslani, F.; Asif, Z.: Properties of ambient-cured normal and heavyweight geopolymer concrete exposed to high temperatures. Mater. 12, 740 (2019). https://doi.org/10.3390/MA12050740
Ul Haq, E.; Kunjalukkal Padmanabhan, S.; Licciulli, A.: Synthesis and characteristics of fly ash and bottom ash based geopolymers-A comparative study. Ceram. Int. 40, 2965–2971 (2014). https://doi.org/10.1016/J.CERAMINT.2013.10.012
Takeda, H.; Hashimoto, S.; Matsui, H.; Honda, S.; Iwamoto, Y.: Rapid fabrication of highly dense geopolymers using a warm press method and their ability to absorb neutron irradiation. Constr. Build. Mater. 50, 82–86 (2014). https://doi.org/10.1016/J.CONBUILDMAT.2013.09.014
Li, Q.; Xu, H.; Li, F.; Li, P.; Shen, L.; Zhai, J.: Synthesis of geopolymer composites from blends of CFBC fly and bottom ashes. Fuel 97, 366–372 (2012). https://doi.org/10.1016/J.FUEL.2012.02.059
Lee, N.K.; Jang, J.G.; Lee, H.K.: Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 53, 239–248 (2014). https://doi.org/10.1016/J.CEMCONCOMP.2014.07.007
Davitovits, J.: Geopolymer Chemistry and Properties.... - Google Akademik. In: Geopolymer Chemistry and Properties
Palomo, A.; Palacios, M.: Alkali-activated cementitious materials: alternative matrices for the immobilisation of hazardous wastes—Part II. Stabilisation of chromium and lead. Cem. Concr. Res. 33, 289–295 (2003). https://doi.org/10.1016/S0008-8846(02)00964-X
Behera, P.; Baheti, V.; Militky, J.; Louda, P.: Elevated temperature properties of basalt microfibril filled geopolymer composites. Constr. Build. Mater. 163, 850–860 (2018). https://doi.org/10.1016/J.CONBUILDMAT.2017.12.152
Omer, S.A.; Demirboga, R.; Khushefati, W.H.: Relationship between compressive strength and UPV of GGBFS based geopolymer mortars exposed to elevated temperatures. Constr. Build. Mater. 94, 189–195 (2015). https://doi.org/10.1016/J.CONBUILDMAT.2015.07.006
Rashad, A.M.: An investigation of high-volume fly ash concrete blended with slag subjected to elevated temperatures. J. Clean. Prod. 93, 47–55 (2015). https://doi.org/10.1016/J.JCLEPRO.2015.01.031
Zhang, H.Y.; Kodur, V.; Qi, S.L.; Cao, L.; Wu, B.: Development of metakaolin-fly ash based geopolymers for fire resistance applications. Constr. Build. Mater. 55, 38–45 (2014). https://doi.org/10.1016/j.conbuildmat.2014.01.040
Sumajouw, D. M. J.; Hardjito, D.; Wallah, S. E.; Rangan, B. V.: Flexural behaviour fly ash based geopolymer concrete beams. In: Proceedings of the 22nd Bienniel concference of the Concrete Institute of Australia. Presented at the (2005)
Rickard, W.D.A.; Kealley, C.S.; Van Riessen, A.: Thermally induced microstructural changes in fly ash geopolymers: experimental results and proposed model. J. Am. Ceram. Soc. 98, 929–939 (2015). https://doi.org/10.1111/JACE.13370
Sarker, P.K.: Bond strength of reinforcing steel embedded in fly ash-based geopolymer concrete. Mater. Struct. (2010). https://doi.org/10.1617/s11527-010-9683-8
Sumajouw, D.M.J.; Hardjito, D.; Wallah, S.E.; Rangan, B.V.: Fly ash-based geopolymer concrete: study of slender reinforced columns. J. Mater. Sci. 42, 3124–3130 (2007). https://doi.org/10.1007/S10853-006-0523-8/TABLES/4
Bakırdöven, A.: Usability and characterization of industrial wastes in geopolymers production. Inonu University Institute of Science Department of Chemical Engineering, M.S. Thesis. (2021)
Kriven, W.M.; Bell, J.L.; Gordon, M.: Microstructure and microchemistry of fully-reacted geopolymers and geopolymer matrix composites. Ceram. Trans. 153, 227–250 (2003)
Fernández-Jiménez, A.; Palomo, A.: Composition and microstructure of alkali activated fly ash binder: effect of the activator. Cem. Concr. Res. 35, 1984–1992 (2005). https://doi.org/10.1016/J.CEMCONRES.2005.03.003
Bernal, S.A.; Provis, J.L.; Walkley, B.; San Nicolas, R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; Van Deventer, J.S.J.: Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 53, 127–144 (2013). https://doi.org/10.1016/J.CEMCONRES.2013.06.007
Hassan, A.; Arif, M.; Shariq, M.: Mechanical behaviour and microstructural investigation of geopolymer concrete after exposure to elevated temperatures. Arab. J. Sci. Eng. 45, 3843–3861 (2020). https://doi.org/10.1007/S13369-019-04269-9
Martin, A.; Pastor, J.Y.; Palomo, A.; Fernández Jiménez, A.: Mechanical behaviour at high temperature of alkali-activated aluminosilicates (geopolymers). Constr. Build. Mater. 93, 1188–1196 (2015). https://doi.org/10.1016/J.CONBUILDMAT.2015.04.044
Torres-Carrasco, M.; Puertas, F.: Waste glass in the geopolymer preparation. Mechanical and microstructural characterisation. J. Clean. Prod. 90, 397–408 (2015). https://doi.org/10.1016/J.JCLEPRO.2014.11.074
Mehta, A.; Siddique, R.: Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 146, 136–143 (2017). https://doi.org/10.1016/J.CONBUILDMAT.2017.04.077
Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Dawood Abdul Khadar, S.: Molarity activity effect on mechanical and microstructure properties of geopolymer concrete: a review. Constr. Mater Case Stud (2022). https://doi.org/10.1016/J.CSCM.2022.E01014
Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J.: The role of inorganic polymer technology in the development of “green concrete.” Cem. Concr. Res. 37, 1590–1597 (2007). https://doi.org/10.1016/J.CEMCONRES.2007.08.018
Zhang, H.; Li, L.; Yuan, C.; Wang, Q.; Sarker, P.K.; Shi, X.: Deterioration of ambient-cured and heat-cured fly ash geopolymer concrete by high temperature exposure and prediction of its residual compressive strength. Constr. Build. Mater. (2020). https://doi.org/10.1016/J.CONBUILDMAT.2020.120924
Fan, F.; Liu, Z.; Xu, G.; Peng, H.; Cai, C.S.: Mechanical and thermal properties of fly ash based geopolymers. Constr. Build. Mater. 160, 66–81 (2018). https://doi.org/10.1016/J.CONBUILDMAT.2017.11.023
Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S.J.: Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 45, 125–135 (2014). https://doi.org/10.1016/J.CEMCONCOMP.2013.09.006
Liu, J.C.; Tan, K.H.; Yao, Y.: A new perspective on nature of fire-induced spalling in concrete. Constr. Build. Mater. 184, 581–590 (2018). https://doi.org/10.1016/J.CONBUILDMAT.2018.06.204
Lahoti, M.; Wong, K.K.; Yang, E.H.; Tan, K.H.: Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 44, 5726–5734 (2018). https://doi.org/10.1016/J.CERAMINT.2017.12.226
Abdulkareem, O.A.; Mustafa Al Bakri, A.M.; Kamarudin, H.; Khairul Nizar, I.; Saif, A.A.: Effects of elevated temperatures on the thermal behavior and mechanical performance of fly ash geopolymer paste, mortar and lightweight concrete. Constr. Build. Mater. 50, 377–387 (2014). https://doi.org/10.1016/j.conbuildmat.2013.09.047
Mejía, J.M.; Rodríguez, E.; Mejía De Gutiérrez, R.; Gallego, N.: Preparation and characterization of a hybrid alkaline binder based on a fly ash with no commercial value. J. Clean. Prod. 104, 346–352 (2015). https://doi.org/10.1016/J.JCLEPRO.2015.05.044
Luhar, S.; Nicolaides, D.; Luhar, I.: Fire resistance behaviour of geopolymer concrete: an overview. Build. 11, 82 (2021). https://doi.org/10.3390/BUILDINGS11030082
Deng, Z.; Yang, Z.; Bian, J.; Lin, J.; Long, Z.; Hong, G.; Yang, Z.; Ye, Y.: Advantages and disadvantages of PVA-fibre-reinforced slag- and fly ash-blended geopolymer composites: engineering properties and microstructure. Constr. Build. Mater. 349, 128690 (2022). https://doi.org/10.1016/J.CONBUILDMAT.2022.128690
Luhar, S.; Chaudhary, S.; Luhar, I.: Thermal resistance of fly ash based rubberized geopolymer concrete. J. Build. Eng. 19, 420–428 (2018). https://doi.org/10.1016/J.JOBE.2018.05.025
Tazune, F.K.; Tchakouté, H.K.; Rüscher, C.H.; Tchekwagep, J.J.K.; Hou, P.: Effects of Fe2O3/SiO2 molar ratios in the Fe-silica on the compressive strengths and microstructural properties of geopolymer materials derived from waste fired clay brick and metakaolin. J. Inorg. Organomet. Polym. Mater. 1, 1–13 (2023). https://doi.org/10.1007/S10904-023-02913-4/FIGURES/15
Temuujin, J.; Minjigmaa, A.; Lee, M.; Chen-Tan, N.; Van Riessen, A.: Characterisation of class F fly ash geopolymer pastes immersed in acid and alkaline solutions. Cem. Concr. Compos. 33, 1086–1091 (2011). https://doi.org/10.1016/J.CEMCONCOMP.2011.08.008
Criado, M.; Fernández-Jiménez, A.; de la Torre, A.G.; Aranda, M.A.G.; Palomo, A.: An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 37, 671–679 (2007). https://doi.org/10.1016/J.CEMCONRES.2007.01.013
Klima, K.M.; Schollbach, K.; Brouwers, H.J.H.; Yu, Q.: Enhancing the thermal performance of Class F fly ash-based geopolymer by sodalite. Constr. Build. Mater. 314, 125574 (2022). https://doi.org/10.1016/J.CONBUILDMAT.2021.125574
Jiang, X.; Xiao, R.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B.: A laboratory investigation of steel to fly ash-based geopolymer paste bonding behavior after exposure to elevated temperatures. Constr. Build. Mater. 254, 119267 (2020). https://doi.org/10.1016/J.CONBUILDMAT.2020.119267
Yavuz, E.; Kul Gul, N.I.; Kockal, N.U.: Characterization of class C and F fly ashes based geopolymers incorporating silica fume. Ceram. Int. 48, 32213–32225 (2022). https://doi.org/10.1016/J.CERAMINT.2022.07.163
Tayeh, B.A.; Hakamy, A.; Amin, M.; Zeyad, A.M.; Agwa, I.S.: Effect of air agent on mechanical properties and microstructure of lightweight geopolymer concrete under high temperature. Case Stud. Constr. Mater. (2022). https://doi.org/10.1016/J.CSCM.2022.E00951
Jo, B.; Park, S.; Park, J.: Properties of concrete made with alkali-activated fly ash lightweight aggregate (AFLA). Cem. Concr. Compos. 29, 128–135 (2007). https://doi.org/10.1016/J.CEMCONCOMP.2006.09.004
Rovnaník, P.; Bayer, P.; Rovnaníková, P.: Characterization of alkali activated slag paste after exposure to high temperatures. Constr. Build. Mater. 47, 1479–1487 (2013). https://doi.org/10.1016/J.CONBUILDMAT.2013.06.070
Hussin, M.W.; Bhutta, M.A.R.; Azreen, M.; Ramadhansyah, P.J.; Mirza, J.: Performance of blended ash geopolymer concrete at elevated temperatures. Mater. Struct. Constr. 48, 709–720 (2015). https://doi.org/10.1617/S11527-014-0251-5/TABLES/4
Ye, J.; Zhang, W.; Shi, D.: Effect of elevated temperature on the properties of geopolymer synthesized from calcined ore-dressing tailing of bauxite and ground-granulated blast furnace slag. Constr. Build. Mater. 69, 41–48 (2014). https://doi.org/10.1016/J.CONBUILDMAT.2014.07.002
Chindaprasirt, P.; Boonbamrung, T.; Poolsong, A.; Kroehong, W.: Effect of elevated temperature on polypropylene fiber reinforced alkali-activated high calcium fly ash paste. Case Stud. Constr. Mater. (2021). https://doi.org/10.1016/J.CSCM.2021.E00554
Acknowledgements
This research project was funded by TUBITAK (The Scientific and Technological Research Council of Turkey) with grant number 121M236. The authors extend their gratitude to the other researchers who contributed to the study.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
HK contributed to the conceptualization, methodology, visualization, investigation and writing—original draft preparation; AÖ was involved in the review, editing, methodology, investigation, resources, project administration and funding acquisition. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kucukgoncu, H., Özbayrak, A. Microstructural Analysis of Low-Calcium Fly Ash-Based Geopolymer Concrete with Different Ratios of Activator and Binder Under High Temperatures. Arab J Sci Eng (2024). https://doi.org/10.1007/s13369-024-09266-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13369-024-09266-1