Abstract
Progress in stem cell research has revolutionized the medical field for more than two decades. More recently, the discovery of induced pluripotent stem cells (iPSCs) has allowed for the development of advanced disease modeling and tissue engineering platforms. iPSCs are generated from adult somatic cells by reprogramming them into an embryonic-like state via the expression of transcription factors required for establishing pluripotency. In the context of the central nervous system (CNS), iPSCs have the potential to differentiate into a wide variety of brain cell types including neurons, astrocytes, microglial cells, endothelial cells, and oligodendrocytes. iPSCs can be used to generate brain organoids by using a constructive approach in three-dimensional (3D) culture in vitro. Recent advances in 3D brain organoid modeling have provided access to a better understanding of cell-to-cell interactions in disease progression, particularly with neurotropic viral infections. Neurotropic viral infections have been difficult to study in two-dimensional culture systems in vitro due to the lack of a multicellular composition of CNS cell networks. In recent years, 3D brain organoids have been preferred for modeling neurotropic viral diseases and have provided invaluable information for better understanding the molecular regulation of viral infection and cellular responses. Here we provide a comprehensive review of the literature on recent advances in iPSC-derived 3D brain organoid culturing and their utilization in modeling major neurotropic viral infections including HIV-1, HSV-1, JCV, ZIKV, CMV, and SARS-CoV2.
Similar content being viewed by others
Three-dimensional (3D) brain organoid cultures
The human brain is the most complex organ in our body, with many aspects of its development and human-specific pathology remaining unknown because of limited accessibility to living human brain tissue. As a result, many approaches and models have been generated in an attempt to recapitulate the human brain in an in vitro system.
Two-dimensional (2D) cell culture systems have historically been used to model various diseases and systems in vitro; however, they are quite limited when it comes to modeling complex systems such as the human brain. 2D-cell culture systems lack the cellular organization that is present in the brain, as they are grown in a monolayer format which limits cell interactions only to their periphery. This results in a lack of proper oxygen and nutrient diffusion as well as waste clearance (Antoni et al. 2015). Furthermore, 2D systems lack the tissue complexity that is present in the brain. As cells are often cultured as a single cell type, they are missing the cell-to-cell interaction between different cell types that is present in vivo. To create a 2D system that is more resemblant to cellular interactions in the human brain, co-cultures of cells can be created, such as co-culturing neurons with other neural cell types such as microglia (Haenseler et al. 2017; Vahsen et al. 2022). Despite these co-culturing techniques, the cells are still limited to peripheral contacts and lack the organization needed to recapitulate the human brain in vitro.
Various neuronal cells, such as primary neurons and neuroblastoma cells, have been used in 2D systems in neuroscience research (Liu et al. 2022). However, obtaining patient-derived brain tissue, or neural stem cells and embryonic stem cells that can differentiate into neuronal cells, is controversial and not always accessible (Gabriel and Gopalakrishnan 2017). The innovation of induced pluripotent stem cell (iPSC) technology eased this problem by opening the doors to being able to generate human neural cell types without the need to isolate cells directly from the human Central Nervous System (CNS) (Gabriel and Gopalakrishnan 2017). The somatic cell-derived iPSCs are a type of stem cell that can differentiate into other cell types in the body (Lyadova and Vasiliev 2022), which makes them an available source for researchers (Karagiannis and Kim 2021). iPSCs were developed by Takahashi and Yamanaka 2006 at Kyoto University in Japan, where they first induced pluripotency in mouse embryonic stem cells (ESCs) and then in adult human fibroblast cells a year later (Takahashi and Yamanaka 2006; Takahashi et al. 2007). Takahashi and Yamanaka generated their iPSCs from fibroblast culture by using retroviral vectors to introduce specific transcription factors such as Oct3/4, Sox2, Klf4, and c-Myc into the skin cells. They noted that their iPSCs display both the morphology and growth properties of ESCs and express ESC-specific marker genes (Takahashi and Yamanaka 2006; Takahashi et al. 2007). Researchers have created techniques for generating iPSCs without using viral vectors, like plasmid-based or episomal reprogramming, that can eliminate the dangers linked to mutations due to viral integration into the genome (Bang et al. 2018). This method involves electroporation, where the electrical shocks introduce plasmid into the genome of the cells. Additionally, CRISPR-based reprogramming allows for the precise and efficient editing of certain genes for successful reprogramming (Liu et al. 2018).
Human iPSCs (hiPSC) prove a powerful tool for easily generating human neural cell types in culture; however, the differentiated cells are still 2D and limited in the same way other 2D cell culture systems are, as listed previously. This led to the need to develop alternative models to study such systems in vitro while mimicking an in vivo environment, such as 3D culture systems. One of the first successful 3D neural systems was that of neurospheres. Neurospheres are 3D cell aggregates of multipotent neural stem cells (NSC) grown in culture, providing a good resource for studying NSCs in vitro (Soares et al. 2021). These clusters of NSCs can then be differentiated into varying cell types, such as neurons and glial cells, all within the same sphere, also known as a neural spheroid (Dingle et al. 2015; Zhou et al. 2016; Pamies et al. 2017). This further allows for a better representation of cell-to-cell interactions in vitro. Although these systems provide a better representation of the brain in vitro than traditional 2D cell culture, they lack complete cellular composition, organization, and complexity of the human brain (Reynolds et al. 1992; Pamies et al. 2017). The human brain has very specific region specificity and cellular organization that is crucial to its function, and this is lacking in neurospheres and neural spheroids as the cells do not organize (Dingle et al. 2015).
Unlike 2D cell culture systems and neurospheres, brain organoids are able to model the human brain at a cellular, structural, and developmental level, allowing researchers to model the human brain and its function in ways that were previously impossible. Brain organoids were first generated by Lancaster et al. in (2013) as a system to study microcephaly. They were able to successfully generate an iPSC-derived 3D cell system, which they dubbed “cerebral organoids” (COs), that displayed discrete brain regions, dorsal cortical organization, functional cortical neurons, and glial cell populations (Lancaster et al. 2013). The development of this system has been a major breakthrough in neural sciences research as it was the first time the human brain was able to be recapitulated in vitro with correct organization and patterning.
To generate organoids, specific conditions, like extracellular matrix (ECM), small molecules, and growth factors, are provided to iPSCs or tissue-derived cells (TDCs) (Zhao et al. 2022). Thus, this environment will differentiate iPSCs or TDCs into the tissue of interest, such as the lung, heart, and cerebral cortex (Zhao et al. 2022). Researchers use stem cells such as iPSCs to generate brain organoids due to their availability (Gabriel and Gopalakrishnan 2017). This method involves differentiating single-cell iPSCs into embryoid bodies (EBs) and then NSCs by using small molecules and growth factors (Hong et al. 2022). Neuroepithelium cells form during the induction phase of EBs (Hong et al. 2022). The expansion phase involves embedding the EBs in ECM such as Matrigel, which results in a budding morphology and promotes further differentiation into several cell types present in COs, such as NSCs, neurons, and glial cells (Agboola et al. 2021). The expanded EBs are cultured in suspension on an orbital shaker (Lancaster and Knoblich 2014) or in a spinning bioreactor (Qian et al. 2016) during and after the maturation phase, where they become self-organized COs.
The organoids generated using these protocols, known as “unguided organoids,” as they are allowed to freely organize themselves into forebrain, midbrain, and hindbrain regions (Lancaster et al. 2013; Qian et al. 2016). This allows for a recapitulation of the entire brain in vitro which is an extremely useful tool; however, some diseases affect specific regions of the brain. As a result, it is necessary to be able to model specifically the forebrain, midbrain, or hindbrain alone, as well as specific structures in the brain as organoids.
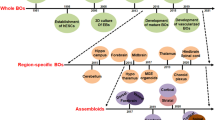
Many groups of researchers have worked to develop guided protocols using extrinsic factors to generate brain region-specific organoids, which contain more accurate cell populations and organization of specific brain regions and structures. A commonly used guided organoid is a cortical organoid, which is representative of the cerebral cortex. Cortical organoids have been used to study a variety of neural disorders, such as Zika virus (ZIKV) infection (Qian et al. 2016), Japanese encephalitis virus (Zhang et al. 2018), Alzheimer’s disease (AD) (Raja et al. 2016), and several other neural degenerative disorders. Beyond cortical organoids, many other brain regions and structures have successfully been generated using guide protocols. These include forebrain and midbrain organoids (Raja et al. 2016; Jo et al. 2016), thalamic and hypothalamic organoids (Xiang et al. 2020; Huang et al. 2021), pituitary organoids (Matsumoto et al. 2020), cerebellar organoids (Ballabio et al. 2020) and hindbrain/brainstem organoids (Eura et al. 2020) (Fig. 1).
Differentiation of different types of human brain organoids from stem cell–derived embryoid bodies using either guided or unguided maturation protocols, as well as assembloid formation (created with biorender.com). Cerebral organoids were stained with H&E and neurons, microglia, astrocytes, and oligodendrocytes were visualized by immunohistochemistry (IHC) TUJ-1/MAP2, IBA1, GFAP, and Olig2 specific antibodies, respectively
These region-specific organoids can be studied alone, but they can also be combined into what is known as an “assembloid” and form an even more complex brain-like structure (Fig. 1). Bagley et al. fused dorsal and ventral forebrain organoids to generate the dorsal–ventral axis and were able to show that these fused organoids can model the complex interactions between different regions of the brain (Bagley et al. 2017). In another study, Xiang et al. fused thalamic organoids with cortical organoids to investigate the circuit organizations and related disorders between the thalamus and cortex (Xiang et al. 2019). Furthermore, Miura et al. generated cortico-striatal assembloids to model complex long-distance forebrain circuits (Miura et al. 2022).
Although brain organoids prove to be a significantly better method for modeling the brain in vitro, they are not without limitations. Most notably, organoids have limited diffusion of nutrients and oxygen to their centers, and as a result, are often limited in size and are prone to cell death within their core (Lancaster et al. 2013). The limited diffusion is likely a result of the lack of a circulatory system within the organoid. In an attempt to overcome this challenge, researchers have attempted to create vascularized organoids though several different methods. One method used successfully by Pham et al. was to embed cerebral organoids with endothelial cells derived from the same line of iPSCs (Pham et al. 2018). Another approach used was to create assembloids between cerebral organoids and brain vascular organoids, which was able to recapitulate several aspects of the blood–brain-barrier (Sun et al. 2022). Additionally, the vascularization of organoids has been done using an in vivo model of transplantation. Mansour et al. transplanted human iPSC-derived brain organoids into adult mice brains and were able to see extensive vascularization, as well as neuronal integration between the organoid and the host brain, and microglial infiltration (Mansour et al. 2018). Vascularization of organoids is still a work in progress; however, there are many promising approaches to improving this system and improving organoids as in in vitro models of the human brain.
Cerebral organoids are often generated by pushing iPSCs into a neuroectoderm lineage by inhibiting the formation of the mesoderm and endoderm. As a result, many organoids used have reportedly lacked microglia, which are derived from the mesoderm and are critical to studying immune responses in the brain. A study done by Dos Reis et al. incorporated microglia into their organoids by integrating human immunodeficiency virus (HIV) infected microglia into 2-week-old organoids (dos Reis et al. 2020, 2023). However, Ormel et al. showed that microglia can innately develop within a CO (Ormel et al. 2018). This represents a valuable resource for studying interactions between microglia, neurons, and other glial cells as well as modeling impacts of immune responses on the brain, such as viral infections.
Neurotropic viral infections have been difficult to accurately study both in vitro and in vivo, as a result of the lack of a multicellular composition in vitro and differences between human and mice brains in vivo. Of importance is that viruses can either directly infect cells to cause an effect, or uninfected cells may be affected indirectly as a result of infected cells releasing cytokines, chemokines, viral proteins, and other toxic factors. This is especially important in looking at viruses such as HIV, which infects microglial cells and possess neurotoxic effects on neurons that are not prone to HIV infection (Kovalevich and Langford 2012). Furthermore, in vivo mice systems do not accurately represent the human brain, and certain viruses such as JC virus (JCV) and HIV-1 cannot infect mice, providing a need for a better human-based system. Therefore, 3D brain organoids may provide an incredibly powerful system for modeling human neurotropic viral infections in vitro.
NeuroHIV modeling in 3D brain organoids
HIV is a retrovirus that enters target cells through the interaction of viral proteins and host cell receptors CD4 and CC-chemokine receptor 5 (CCR5) or CXC-chemokine receptor 4 (CXCR4) (Deeks et al. 2015). Shortly after initial infection, HIV enters the CNS and establishes viral reservoirs leading to neuropathogenesis (Enting et al. 2001; Zayyad and Spudich 2015). In the brain, microglia are the major cell type infected by HIV, while infection of astrocytes remains controversial even though they may play a key role in neuropathogenesis (Brack-Werner 1999; Wallet et al. 2019). Neurons are typically not infected with HIV but can be injured by indirect mechanisms, such as toxic viral proteins and neurotoxicity from glial activation (Kovalevich and Langford 2012). Early in the HIV/AIDS pandemic, pre combination antiretroviral therapy (cART) era, an estimate of 20–30% of HIV patients developed HIV-associated dementia (HAD) (González-Scarano and Martín-García 2005). With the advent of cART, the incidence of HAD has significantly decreased, although a large percentage of people with HIV (PWH) still develop neurological disorders, grouped into the terms of HIV-associated neurocognitive disorders (HAND) (Antinori et al. 2007; Clifford and Ances 2013). HAND manifestation ranges from asymptomatic neurocognitive impairment (ANI) to mild neurocognitive disorder (MND) and HIV-associated dementia (HAD) (Clifford and Ances 2013).
Understanding the neuropathology of HIV is particularly important for elucidating mechanisms associated with cognitive impairment seen in PWH. One of the major obstacles in studying HIV neuropathogenesis is the lack of in vitro culture models which accurately recapitulate HAND, as multiple CNS cell types may contribute to the pathology. Additionally, animal models have been used in the past, such as nonhuman primates (NHP) or genetically modified mouse models. Although these animal models have helped researchers understand many aspects of HIV pathophysiology, there are still some limitations in the understanding of HIV-CNS interactions contributing to neurocognitive disorders (Mallard and Williams 2018). In humans, studies on HIV neuropathology have been limited to the collection and analysis of post-mortem brain tissues. The majority of the studies on neuroHIV in vitro have been performed in 2D culture models, using immortalized microglial cell lines, peripheral blood monocyte-derived microglia (MMG), or primary human microglia isolated from human tissues (Garcia-Mesa et al. 2017; Rawat and Spector 2017; Rai et al. 2020). The recent advances in iPSC culture research and the generation of brain organoids have allowed the creation of 2D and 3D in vitro models for studying neuroHIV in humans.
HIV modeling in human brain organoids has recently been described by two groups (dos Reis et al. 2020; Gumbs et al. 2022) with a potential to be adopted and further developed by the neuroHIV research community. These two groups used two different approaches to develop human brain organoids containing microglia, the major cell type along with macrophages responsible for HIV infection and replication in the brain (Wiley et al. 1999). Dos Reis and colleagues developed a model of 3D human brain organoids (hBORG) containing microglia (MG) which were infected with HIV prior to their integration into hBORGs. Neurospheres from neuronal progenitor cells (NPCs) were first generated using a media containing a combination of astrocyte differentiation media and neuronal media. The hBORGs generated contained only neurons and astrocytes. HMC3 cells, an immortalized human microglia cell line, were first infected with a neurotropic HIV-1 (NL(YU2-Env)-EGFP) reporter virus and then placed on top of the hBORGs. The authors showed that the majority of microglia were on the outer layer of the hBORGs, with some migrated and embedded into the organoids. In addition, HIV-infected or control (uninfected) primary human microglia from post-mortem adult human brain tissues were combined with hBORGs. Interestingly, HIV-infected human microglial cells showed some level of infiltration into the hBORGs and established productive infection. Moreover, both HMC3 and primary microglia containing hBORGs infected with HIV showed higher levels of TNF-α and IL-1β in culture supernatant compared to mock-infected MG-hBORGs. This suggested recapitulation of HIV-1 CNS pathology trademarks from post-mortem brain tissues from HIV-infected individuals (at some degree) in the MG-hBORG model. While evaluating cell toxicity in infected primary MG-hBORGs, an increase in neuronal cell death was also observed. In addition, a decrease in βIII-Tubulin (neurons) mRNA and an increase in GFAP (astrocytes) expression was observed, suggesting neuronal loss and astrocytosis, two of the characteristics of neuropathology associated with severe HAND (Huangui Xiong 2013). Although these MG-hBORG models provided advancement in the modeling of HIV infection and recapitulated some aspects of HIV CNS disease, they also possessed some limitations. Primarily, the limited incorporation of exogenously added microglial cells into organoids does not truly represents the in vivo conditions. The endogenous and natural distribution of microglia with properly developed interactions with other cell types including neurons and astrocytes within the organoids would provide a better representation of neuroHIV pathophysiology. This was recently achieved by Gumbs and colleagues (Gumbs et al. 2022) utilizing a microglia-containing 3D brain organoid model which was established earlier by the same group (Ormel et al. 2018). COs were generated from iPSCs with cells from the three germ layers at early stages during the induction and expansion phases. In particular, the formation of mesodermal progenitors with the potential to generate microglial cells was a great advancement for the development of COs with microglial incorporation. Indeed, analysis of cell-specific markers revealed that the COs contained neurons, astrocytes, and microglial cells along with CD4 and CCR5 receptor expressions in microglial cells. To evaluate the HIV-1 infectivity of these cells, authors isolated organoid-derived microglia (oMG) and infected them with CCR5 M-tropic HIV1_bal-luciferase and HIV-GFP reporter viruses along with human primary microglia (pMG) cells. As anticipated, both oMG and pMG cells supported HIV-1 infection. Once the viral infection was confirmed in CO-derived microglial cells, HIV-1 infection in COs was characterized. Interestingly, one interesting observation was the Matrigel interference of HIV infections in COs, suggesting that modeling of HIV in COs may need further improvements for reliable and reproducible infection outcomes. In addition, COs from different iPSC lines showed variations in susceptibility to HIV infection. Expression of HIV receptors, such as CD4, CXCR4, and CCR5, were also variable between different organoids. Nonetheless, the CO model of HIV infection described by Gumbs et al. provides a unique platform (although further improvements are needed) to better understand cellular and viral infections in a 3D culture system in vitro.
Human-derived brain organoid models of Herpesvirus type 1
Herpesvirus type 1 (HSV-1) belongs to the Herpesviridae family (Alphaherpesvirinae subfamily) and consists of a double-stranded DNA genome of ~ 152 kbp, which encodes for ~ 80 viral proteins (Denes et al. 2020). The worldwide prevalence of HSV-1 infection in adolescents and adults is between 45 and 90%, and the primary infection usually occurs during childhood with the establishment of latent infection in sensory neurons. The primary infection sites for HSV-1 are mostly the skin and mucous membranes of the lips and less frequently the genital mucosa. After infection, viral particles retrogradely move through the axons of ganglionic neurons to where the viral DNA genome is assembled into a heterochromatin-like structure. As neurons are non-dividing and they are not susceptible to immunological surveillance, HSV-1 infection in neurons results in a lifelong lasting infection. HSV-1 can also cause herpetic keratitis, which results in corneal scarring and impairment of sight. The most detrimental HSV-associated disease is herpes encephalitis, a rare disease that affects 2–4 out of 1,000,000 people annually with high mortality rates, that occurs when the virus reaches the CNS (Bradshaw and Venkatesan 2016). Furthermore, pregnant women are at risk of mother-to-child transmission of genital herpes, which may lead to fetal encephalitis and perinatal mortality if an emergency cesarean delivery is not performed (Kimberlin 2004; Messacar et al. 2018). Pathophysiological features of HSV-1-associated neurological disorders remain unclear, impelling researchers to establish relevant in vitro models of human CNS to understand the molecular mechanism of viral latency, reactivation, and neuropathology.
HSV-1 infection has been primarily studied in either human- or non-human- derived primary or immortalized cell cultures and cell lines. However, the obtained results in many cases possess reliability and reproducibility issues due to the variations in the genetic assets of the cell lines employed in different experiments. Moreover, testing antiviral drug candidates on cell lines has often been inconsistent. Therefore, in most pre-clinical studies, the efficacy of HSV-1 vaccines has been evaluated in mice models. These in vivo models have provided useful information on immune response against primary HSV-1 infection at the expense of the vaccine’s efficacy against recurrent viral shedding and disease events, which are both extremely rare in mice (Gebhardt and Halford 2005). The lack of an animal model that translates human immune responses to viral infection still represents a major limitation to develop valid therapeutic vaccines (Dasgupta and BenMohamed 2011). The emergence of stem cell-based technologies has led to the rapid development of human in vitro model systems which may provide a needed platform for pre-clinical HSV-1 studies.
The first study where hiPSC-derived 2D and 3D neuronal models were used to investigate the basic features of HSV-1 infection was recently reported (D’Aiuto et al. 2019). This study has provided convincing evidence of HSV-1 replication and latency establishment in hiPSC-derived CNS neurons. The latency induction protocol enlisted a combination of alpha interferon (IFN-a) and (E)-5-(2-bromovinyl)-2'-deoxyuridine (5BVdU) as antivirals. In the 2D culture, viral reactivation was induced by histone deacetylase (HDAC) inhibitors. During the latency induction, the culture conditions drove viral chromatin into a heterochromatic state, increased the expression of latency-associated transcript (LAT), and the presence of the transcriptional corepressor KAP1 in the ICP4 promoter region which was also shown for the latency of Kaposi’s sarcoma-associated herpesvirus (KSHV) (King et al. 2015). On the other hand, in 3D culture of scaffold-free brain organoids, HSV-1 LAT expression increased as the latency was established, but spontaneous reactivation was observed in approximately 17% of the organoids between days 8 and 11 after infection and antiviral removal, followed by neuronal morphological changes, such as degeneration of neuronal processes and neuronal syncytia formation. HSV-1 reactivation has been shown to occur infrequently in this 3D model compared to the cognate 2D model, possibly due to the differences in cell-to-cell and cell-to-extracellular matrix interactions observed between these two models. In 2020, the same research team (Zheng et al. 2020) employed hiPSCs to investigate HSV-1 infection of NPCs when they are cultured as 2D monolayers or as 3D neurospheres, starting from the hypothesis that the neurons acquire their exclusive features of HSV-1 latency sites during the neuronal differentiation of NPCs. As reported in their previous study (D’Aiuto et al. 2019), the 3D neurospheres have shown a reduced susceptibility to HSV-1 compared to the 2D NPC monolayer. Furthermore, comparative gene expression analysis showed a significant reduction of LAT expression and viral gene expression in infected NPCs (in 2D and 3D culture) exposed to 5BVdU/IFN-a compared to acutely infected cells. These expression results were comparable with those observed in CNS neurons in vivo (Gussow et al. 2006) but not in neurons of the sensory ganglia. Moreover, this study indicated that the silenced viral genomes in NPCs may not respond to the phosphoinositide 3-kinase inhibitor (PI3Ki). In addition to hiPSC-derived organoids, microfluidic chips in combination with organoids represent a distinct approach based on bioengineering strategies that started being widely employed as platforms that mimic the complexity of organs. Mazzara and colleagues (Mazzara et al. 2022), have recently developed an in vitro human 3D cellular system on microfluidic chips to simulate the connectivity between different human sensory neurons and peripheral tissues, using hiPSC-derived dorsal root ganglia organoids (DRGO) and monolayers of keratinocytes, providing a powerful in vitro model of HSV-1 latency and reactivation. The DRGO could be productively infected with HSV-1 due to the presence of several specific HSV receptors, confirmed by a whole transcriptome profile. Primary infection using the low-titer virus in a medium supplemented with Acyclovir (ACV) has led to the latency establishment in DRGO, and the two stages of infection (latency and productive infection) have been confirmed by analyzing LAT and late genes. Moreover, the use of recombinant HSV-1 virus, where reported genes of green fluorescent protein (GFP) and red fluorescent protein (RFP) were cloned under the promoters of the latent viral gene ICP0 and the lytic viral gene of glycoprotein C respectively, has allowed live imaging discrimination of viral latency or reactivation state (Thompson et al. 2003). In this experiment, green fluorescence and LAT transcripts were detected in DRGO, whereas human primary keratinocytes (NHEK) remained not infected, showing that infective viral particles could not passively diffuse from DRGO to keratinocytes. Only thermal stress has led to virus reactivation in DRGO, and both immunofluorescence and gene expression analysis have shown productive infection in both chip chambers, leading the researcher of this study to conclude that HSV-1 have spread from DRGO to NHEK only through an axonal anterograde route. The microfluidics chip technology represents a promising tool to study the molecular mechanisms involved in neurotropic virus infections and a suitable platform for drug screening, treatment responses, and/or therapeutics validation, which otherwise could not be fully investigated by other in vitro models.
During the last decade, several discoveries have emerged showing a strong correlation of HSV-1 infection with beta-amyloid (Aβ) associated neuropathologies, such as AD (Nilsson et al. 2010; Li Puma et al. 2019). In general, the Aβ balance in the human brain is maintained through three key checkpoints: (1) the Aβ generation, (2) the transport of Aβ across the blood–brain-barrier (BBB), and (3) the Aβ degradation (Nilsson et al. 2010). Apolipoprotein E (APOE), the main cholesterol transporter in the brain, participates in both the Aβ transport across the BBB (Linard et al. 2020) and the Aβ degradation (Baranello et al. 2015). Notably, APOE4 expression has been shown to correlate with frequent reactivations of HSV-1 and an increase in AD risk (Linard et al. 2020). Cairns et al. (Cairns et al. 2020) have pioneered a 3D bioengineered brain model by seeding NPCs into a silk protein scaffold-based microdevice to study the effects of HSV-1 infection on AD pathology. Using this bioengineered brain model, it was found that HSV-1 infection could determine the onset of AD-like phenotypes, including Aβ formation, neuroinflammation, gliosis, and diminished neural network functionality. However, this model had limitations in cortical cellular diversity and spatiotemporal self-organization in the human cerebral cortex, as observed in COs. More recently, using 2D neuron cultures and 3D organoids, it was reported that HSV-1-infected COs could be a potential model of AD pathophysiology with the recapitalization of many AD hallmarks including beta-amyloid deposition, dysregulated AD mediators, neuroinflammation, and impaired neuronal differentiation (Qiao et al. 2022). In addition, AD mediator genes PSEN1, PSEN2, Achaete-scute homolog (ASCL), and erythropoietin-producing human hepatocellular receptors B (EPHB) were shown to be significantly upregulated in COs, whereas BACE expression was decreased in response to the HSV-1 infection. Moreover, in the same study, Valacyclovir (VCV) and Ribavirin (RBV) have been reported to significantly reduce HSV-1 replication and rescue the HSV-1-induced pathologic phenotypes in COs. In an earlier study, HSV-1-infected 2D monolayer neuronal cultures presented perinuclear Aβ42 in ICP4-positive cells compared to uninfected cells or infected cells exposed to 5BVdU and IFN-a, supporting the ability of HSV-1 to trigger Aβ42 accumulation (Abrahamson et al. 2021). On the other hand, when 3D brain organoids were infected with HSV-1, Aβ42 immunoreactivity was primarily observed in ICP4-negative neurons and rarely in ICP4-positive neurons, which was mainly linked to the strong antiviral signaling in abortively infected cells. These observations suggested that 3D brain organoids may provide a superior in vitro platform for AD compared to 2D cultures, with much closer translational outcomes to in vivo conditions.
HSV-1 infection has been also shown to be associated with neonatal encephalitis during pregnancy, delivery, and/or postnatally. Intrauterine HSV-1 infection represents approximately 4–5% of HSV infections in newborns (Marquez et al. 2011), and in many cases, it evolves into a fatal neurologic disorder (Straface et al. 2012; Pichler et al. 2015). D’Aiuto and colleagues (D’Aiuto et al. 2022) developed a reductionist approach to model neurodevelopmental outcomes of HSV-1 infection of neural rosettes, which represents the in vitro equivalent of differentiating neural tubes (Broccoli et al. 2014). Early-stage brain organoids (ES-organoids) composed of hiPSCs-derived neural rosettes were employed to investigate aspects of the potential neuro-pathological effects of HSV-1 infection on neurodevelopment. ES-organoids were infected with HSV-1 and treated with ACV for an extended time period (9–12 weeks), which is necessary to generate brain organoids that exhibit cortical-like structures. Interestingly, despite the antiviral treatment, changes in neural rosettes, loss of structural integrity, and neuronal alterations were observed in HSV-1-infected ES-organoids. Qiao and colleagues (Qiao et al. 2020) also reported an in vitro neurodevelopmental model utilizing COs and showed that HSV-1 infection dysregulates neurogenesis by decreasing the expression levels of Nestin, hindbrain markers (ISL1) and reducing the cortical plate layer depth, suggesting that HSV-1-induced dysregulation of brain regionalization may occur in different stages of fetal brain development.
In recent years, iPSCs have offered an appealing opportunity to generate individual specific CNS cells from relevant diseases. However, the variability in the interaction of HSV-1 with donor-specific cell types has not been investigated in depth. Prior studies have been limited to the modeling of rare single-gene inborn errors of immunity in hiPSC-derived CNS cells from human fibroblasts (Lafaille et al. 2012; Zimmer et al. 2018; Chansard et al. 2021). A recent study (Zheng et al. 2022) compared the effects of HSV-1 infection at low multiplicity of infection on the proliferation and migration of two hiPSC-derived NPC lines from a healthy woman (02SF) and her son (01SD). HSV-1 was reported to affect the proliferative activity of 02SF and 01SD NPCs in a viral dose-dependent trend, with a difference in the transcriptional activity of HSV-1 genes. The ACV treatment could efficiently counteract the inhibitory action of the virus on NPC proliferation in 02SF but not in 01SD, where NPCs migration was also reduced when compared to 02SF NPCs. The authors concluded that the sensitivity to ACV and the impact that HSV-1 has on neurogenesis are subject to inter-individual differences, explaining why some individuals reactivate HSV more efficiently and why HSV-1 infections in the CNS may have pathological consequences for some individuals and not for others. Krenn and colleagues (Krenn et al. 2021) have also studied the effects of ZIKV and HSV-1 on neurodevelopment in a brain organoid model and showed that both viruses infect human NPCs and cause a reduction in organoid size consistent with other organoid studies (Qiao et al. 2020; D’Aiuto et al. 2022). This work also highlighted the major differences in the cellular innate responses against ZIKV and HSV-1.
Human-derived brain organoid model of Polyomavirus JC infection
JCV infection of oligodendrocytes causes progressive multifocal leukoencephalopathy (PML), a fatal neurodegenerative disease primarily affecting immunosuppressed individuals and characterized by multifocal loci of demyelinating lesions in the white matter (Weissert 2011; Cortese et al. 2021). While more than 90% of the worldwide adult population is infected with JCV (Weissert 2011), PML remains a rare co-morbidity. JCV is a non-enveloped double-stranded DNA virus that enters the host cells through the primary encounter of sialic acid moieties on glycoproteins of the cellular membrane, followed by clathrin-mediated endocytosis (Ferenczy et al. 2012). Viral replication occurs in the cellular nucleus, whereas the assembly of viral particles takes place in the cytoplasm and depends on host cellular pathways of DNA replication and protein folding (Ferenczy et al. 2012). JCV mainly infects oligodendrocytes (Barth et al. 2016), but it may also infect neurons, causing JC virus encephalopathy and JC virus granule cell neuronopathy (Miskin and Koralnik 2015). In vitro cells that support JCV replication include human fetal glial cells (Padgett et al. 1971; Major et al. 1985; Mandl et al. 1987), human embryonic stem cell-derived oligodendrocyte progenitor cells (Schaumburg et al. 2008), human embryonic kidney cells (Miyamura et al. 1980), glial progenitor cells, progenitor-derived astrocytes, and primary astrocytes (Major and Vacante 1989; Messam et al. 2003; Ferenczy et al. 2013). JCV is a species-specific virus that can only infect humans. Inoculation of JCV into mice or hamsters has resulted in tumor formation with no lytic infection and PML development (Barth et al. 2016). This peculiar infectivity of the virus has interfered with the development of in vivo animal models of PML, delaying the study of mechanisms which underly the viral pathogenesis and modalities for antiviral agents. A humanized mouse model was developed in 2014 by implanting human glial progenitor cells into immunodeficient and myelin-deficient mice (Kondo et al. 2014). However, JCV infection in this model resulted in virus replication mainly in human astrocytes with signs of demyelination not imputable to viral lytic infection due to the limited human oligodendrocyte incorporation. Recently, Barreras and colleagues (Barreras et al. 2022) have established a promising human brain organoid model to study JCV infection. The brain organoids consisted of neurons (70%), astrocytes (20%) and oligodendrocytes (10%) with a diameter of 300–350 μm. JCV productively infected astrocytes and oligodendrocytes in the 3D brain organoids with viral nuclear inclusions observed by immunohistochemistry and electron microscopy. The human-derived brain organoid model of JCV may further advance our understanding of viral pathogenesis and allow a high-throughput platform for screening different therapeutic compounds.
ZIKV, CMV, and SARS-CoV-2 modeling in 3D brain organoids
ZIKV is a Flavivirus with an envelope and single-strand RNA genome (Lindenbach and Rice 2003). Since 2015, ZIKV has gained interest following an outbreak in Brazil where infection during pregnancy was associated with microcephaly in newborns (Mlakar et al. 2016). Due to its influence on brain development, in vitro brain organoid models have been developed by many investigators and provided invaluable knowledge on ZIKV pathophysiology. Majority of the studies investigating ZIKV infection in brain organoids utilized unguided protocols to generate the brain organoids, with only a few utilizing guided protocols. ZIKV infection has been shown to impact the growth and size of forebrain organoids as well as the thickness of the ventricular zone layer (Qian et al. 2016; Xu et al. 2019). In addition, forebrain organoids have also been used as a platform to screen the anti-ZIKV activity of small molecule inhibitors (Xu et al. 2016; Li et al. 2020, 2022). Infection studies in several brain organoid models have suggested that ZIKV can replicate in NPCs, astrocytes, and neurons that define the ZIKV neuropathology by mainly resulting in a general size reduction of organoids due to the defective differentiation/maturation and inducing cellular death (Garcez et al. 2016; Cugola et al. 2016; Dang et al. 2016; Wells et al. 2016; Gabriel et al. 2017; Zhou et al. 2017; Salick et al. 2017; Liu et al. 2019; Krenn et al. 2021). In conclusion, brain organoids possess a superior in vitro model to study ZIKV infection, allowing researchers to better understand the viral pathophysiology and utilize them as a platform to screen therapeutic interventions.
Another neurotropic virus that has been associated with microcephaly is the human Cytomegalovirus (CMV). CMV belongs to the Herpesviridae family and is a double-stranded DNA virus that can infect people of all ages. Although the majority of CMV infections are asymptomatic, newborns with congenital CMV infection may present neurologic anomalies such as lethargy and microcephaly (Boppana et al. 2013). Several groups have reported CMV infection modeling in brain organoids using unguided protocols. CMV infection in brain organoids disrupts organoid morphology, probably due to the induced cell death leading to size reduction (Sun et al. 2020). In addition, infection with CMV interferes with neurogenesis and the formation of neural rosette formation from NPCs (Sison et al. 2019). Furthermore, neural signaling, as well as networks important for neurodevelopment, has been shown to be downregulated following CMV infection in brain organoids (O’Brien et al. 2022). Moreover, brain organoids were also modeled for testing the therapeutic potential of neutralizing antibodies for the treatment of newborns with congenital CMV infection (Sun et al. 2020).
In the past three years, the COVID-19 pandemic has been a major health problem worldwide. Although the major target of SARS-CoV-2 is the respiratory system, the viral tropism involves multi-organ systems (Liu et al. 2021). Neurological manifestations in COVID-19 patients have been reported, although the neuropathology associated with SARS-CoV-2 is yet to be elucidated (Mao et al. 2020; Douaud et al. 2022; Ng et al. 2023). In recent years, several brain organoid models have been developed as a tool to gain insight into SARS-CoV-2 neuroinvasion in the CNS, using guided and unguided protocols. Yi et al. reported persistent ACE2 expression during the development of dorsal forebrain organoids from hESCs and showed susceptibility to infection using a SARS-CoV-2-pseudovirus (Yi et al. 2020). Zhang et al. also reported that ACE2, TMPRSS2, and coronavirus entry-associated proteases (cathepsin L, and furin) are readily available in hNPCs and brain organoids generated from hiPSCs and showed that the brain organoids support SARS-CoV-2 infection and replication (Zhang et al. 2020). Jacob et al. have further investigated SARS-CoV-2 infection in region-specific hiPSCs-derived brain organoids. Using cortical, hippocampal, hypothalamic, and midbrain organoids, they reported limited infection in neurons and astrocytes, but robust infection in choroid plexus (ChP) epithelial cells. Moreover, they developed ChP organoids from hiPSCS and reported productive SARS-CoV-2 infection associated with cell death (Jacob et al. 2020). Several other groups also reported SARS-CoV-2 infection modeling in dorsal cortical organoids or cortical organoids. Those studies revealed that SARS-CoV-2 can infect glial cells (McMahon et al. 2021; Andrews et al. 2022), ChP cells (McMahon et al. 2021), and neuronal cells (Zhang et al. 2020; Song et al. 2021). Mesci et al. showed that infections in neurons promote cell death with the loss of excitatory synapses. Moreover, the same group showed that the antiviral drug Sofosbuvir inhibits SARS-CoV-2 replication in a model of cortical organoids (Mesci et al. 2022). Hou et al. also utilized forebrain and midbrain organoids to study replication efficiency and neurotropism of different variants of the virus (SARS-CoV-2 WT, Delta, Omicron BA.1 and Omicron BA.2) and demonstrated a higher replication efficiency of variant Omicron BA.2 with positive viral infection in dopaminergic neurons in midbrain organoids and cortical neurons in forebrain organoids (Hou et al. 2022). Unguided brain organoids have also been developed to mainly demonstrate the virus’s cellular tropism. In line with findings using guided protocols, various neural cell types were shown to be susceptible to infection with SARS-CoV-2 (Bullen 2020; Ramani et al. 2020; Tiwari et al. 2021). Another group showed that SARS-CoV-2 infection in neurons was boosted by the presence of astrocytes in brain organoids which also led to synaptic loss and neuronal toxicity (Wang et al. 2021). Infection in astrocytes was also linked to the promotion of neuronal death (Kong et al. 2022). Pellegrini et al. reported SARS-CoV-2 infection in ChP with limited neuronal infection, probably due to the higher expression levels of ACE2 in this cell type (Pellegrini et al. 2020). Moreover, by using brain organoid models, Samudyata et al. showed SARS-CoV-2 nucleocapsid protein expression in PAX6, MAP2, GFAP, SOX10, OLIG2, and Iba1 positive cells, and revealed a role of microglia in infected organoids in increasing the engulfment of postsynaptic termini with increased phagocytosis and synapse elimination (Samudyata et al. 2022). In summary, brain organoid models have provided a superior platform to investigate neuronal susceptibility, disease mechanisms, and treatment strategies for SARS-CoV-2 infection.
Future advancements
As far as COs have come in recent years, there is still much improvement to be done. One of the biggest hindrances in CO models is the fact that they are size-restricted due to the lack of nutrient and oxygen diffusion to their centers. An improvement in this area would allow for the generation of larger, more complex, and more stable COs and organoids in general. This area is currently being explored in a variety of ways, such as bioengineering special devices to increase diffusion and organoid size (i.e., spinning bioreactors and microfluidic chips) (Lancaster et al. 2013; Kim et al. 2015; Qian et al. 2016; Karzbrun et al. 2018). Another method for improving the diffusion of nutrients and oxygen in COs is to establish vascularization within the organoids. Not only would vascularization increase nutrient diffusion throughout the CO, but it would allow for the addition of a BBB component, which is extremely important in modeling neurovirology and is lacking in brain COs (Miller et al. 2012). As mentioned previously, several attempts at vascularization in organoids have been made through various means and with various degrees of success. Methods such as endothelial cell co-culture and assembloids between vascular organoids and COs have been used with relative levels of success (Pham et al. 2018; Sun et al. 2022). However, these protocols are tedious and complicated and are not yet a reliable and feasible way to generate vascularized COs. Thus, creating reliable and reproducible methods to generate vascularized COs is an area that needs to be explored to generate more accurate models of neuroviral infections, and of the human brain modeling in general. There are also reported variations between COs due to a variety of factors. Donor cell variability due to genetic background and sex differences needs to be further characterized, as they can lead to large differences between different lines of iPSCs (Burrows et al. 2016; Volpato and Webber 2020). Additionally, there can be quite a large batch variability between COs generated from the same line of iPSCs. Batch variability from the same iPSC lines may be a result of handling, source of growth factors, media, equipment, and differences in protocols. Further improvements in protocols and techniques are needed to improve the reliability, reproducibility, and batch variations. Furthermore, new methods for measuring the electrophysiology of COs may be explored through means such as an optimized multi-electrode array. The CO models of the human brain is still a new era of modeling neurotropic viral infections and there is an endless number of possibilities that can and should be explored to improve these systems.
Conclusions
3D brain organoid models are clearly a superior in vitro platform over 2D-cell cultures with their tissue-cell complexities comparable to in vivo conditions. Human brain organoids are able to represent the human brain at the cellular, structural, and developmental levels, allowing researchers to model neurotropic viral infections in ways that were previously not possible. Brain organoids are developed by “unguided protocols,” allowing them to freely organize into the forebrain, midbrain, and hindbrain or “guided protocols” for generating organoids representing specific brain regions of interest (Fig. 1). Over the last decade, many groups of researchers have developed various types of brain organoids and modeled major neurotropic viral infections (Table 1). As we reviewed above, brain organoid models have provided invaluable knowledge towards a better understanding of molecular regulation of neurotropic viral infections and cellular responses. Although they are proven to be a significantly better platform for modeling the brain in vitro, brain organoids also possess some limitations. They are limited in size and prone to cell death at their center due to the limited diffusion of nutrients and oxygen. Improvements with vascularization attempts are in progress with promising outcomes and the biotechnology for 3D modeling of brain organoids is a fast-growing research era. Nonetheless, 3D brain organoids have shown their potential to take the in vitro culture systems to a new level and have allowed for better modeling of neurotropic viral infections.
Data Availability
The data generated during the current study are available from the corresponding author on reasonable request.
References
Abrahamson EE, Zheng W, Muralidaran V et al (2021) Modeling Aβ42 accumulation in response to herpes simplex virus 1 infection: 2D or 3D? J Virol 95:e02219–20, JVI.02219–20. https://doi.org/10.1128/JVI.02219-20
Agboola OS, Hu X, Shan Z et al (2021) Brain organoid: a 3D technology for investigating cellular composition and interactions in human neurological development and disease models in vitro. Stem Cell Res Ther 12:430. https://doi.org/10.1186/s13287-021-02369-8
Andrews MG, Mukhtar T, Eze UC et al (2022) Tropism of SARS-CoV-2 for human cortical astrocytes. Proc Natl Acad Sci USA 119:e2122236119. https://doi.org/10.1073/pnas.2122236119
Antinori A, Arendt G, Becker JT et al (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799. https://doi.org/10.1212/01.WNL.0000287431.88658.8b
Antoni D, Burckel H, Josset E, Noel G (2015) Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci 16:5517–5527. https://doi.org/10.3390/ijms16035517
Bagley JA, Reumann D, Bian S et al (2017) Fused cerebral organoids model interactions between brain regions. Nat Methods 14:743–751. https://doi.org/10.1038/nmeth.4304
Ballabio C, Anderle M, Gianesello M et al (2020) Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat Commun 11:583. https://doi.org/10.1038/s41467-019-13989-3
Bang JS, Choi NY, Lee M et al (2018) Optimization of episomal reprogramming for generation of human induced pluripotent stem cells from fibroblasts. Animal Cells and Systems 22:132–139. https://doi.org/10.1080/19768354.2018.1451367
Baranello R, Bharani K, Padmaraju V et al (2015) Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. CAR 12:32–46. https://doi.org/10.2174/1567205012666141218140953
Barreras P, Pamies D, Monaco MC et al (2022) A human-derived 3D brain organoid model to study JC virus infection. J Neurovirol 28:17–26. https://doi.org/10.1007/s13365-022-01062-7
Barth H, Solis M, Kack-Kack W et al (2016) In vitro and in vivo models for the study of human polyomavirus infection. Viruses 8:292. https://doi.org/10.3390/v8100292
Boppana SB, Ross SA, Fowler KB (2013) Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 57:S178–S181. https://doi.org/10.1093/cid/cit629
Brack-Werner R (1999) Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1–22. https://doi.org/10.1097/00002030-199901140-00003
Bradshaw MJ, Venkatesan A (2016) Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics 13:493–508. https://doi.org/10.1007/s13311-016-0433-7
Broccoli V, Giannelli SG, Mazzara PG (2014) Modeling physiological and pathological human neurogenesis in the dish. Front Neurosci 8. https://doi.org/10.3389/fnins.2014.00183
Bullen CK (2020) Infectability of human brainsphere neurons suggests neurotropism of SARS-CoV-2*. ALTEX. https://doi.org/10.14573/altex.2006111
Burrows CK, Banovich NE, Pavlovic BJ et al (2016) Genetic variation, not cell type of origin, underlies the majority of identifiable regulatory differences in iPSCs. PLoS Genet 12:e1005793. https://doi.org/10.1371/journal.pgen.1005793
Cairns DM, Rouleau N, Parker RN et al (2020) A 3D human brain–like tissue model of herpes-induced Alzheimer’s disease. Sci Adv 6:eaay8828. https://doi.org/10.1126/sciadv.aay8828
Chansard A, Dubrulle N, Poujol de Molliens M et al (2021) Unveiling interindividual variability of human fibroblast innate immune response using robust cell-based protocols. Front Immunol 11:569331. https://doi.org/10.3389/fimmu.2020.569331
Clifford DB, Ances BM (2013) HIV-associated neurocognitive disorder. Lancet Infect Dis 13:976–986. https://doi.org/10.1016/S1473-3099(13)70269-X
Cortese I, Reich DS, Nath A (2021) Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol 17:37–51. https://doi.org/10.1038/s41582-020-00427-y
Cugola FR, Fernandes IR, Russo FB et al (2016) The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534:267–271. https://doi.org/10.1038/nature18296
D’Aiuto L, Bloom DC, Naciri JN et al (2019) Modeling herpes simplex virus 1 infections in human central nervous system neuronal cells using two- and three-dimensional cultures derived from induced pluripotent stem cells. J Virol 93:e00111-e119. https://doi.org/10.1128/JVI.00111-19
D’Aiuto L, Caldwell JK, Wallace CT et al (2022) The impaired neurodevelopment of human neural rosettes in HSV-1-infected early brain organoids. Cells 11:3539. https://doi.org/10.3390/cells11223539
Dang J, Tiwari SK, Lichinchi G et al (2016) Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19:258–265. https://doi.org/10.1016/j.stem.2016.04.014
Dasgupta G, BenMohamed L (2011) Of mice and not humans: How reliable are animal models for evaluation of herpes CD8+-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine 29:5824–5836. https://doi.org/10.1016/j.vaccine.2011.06.083
Deeks SG, Overbaugh J, Phillips A, Buchbinder S (2015) HIV Infection Nat Rev Dis Primers 1:15035. https://doi.org/10.1038/nrdp.2015.35
Denes CE, Everett RD, Diefenbach RJ (2020) Tour de herpes: cycling through the life and biology of HSV-1. Methods Mol Biol 2060:1–30. https://doi.org/10.1007/978-1-4939-9814-2_1
Dingle YT, Boutin ME, Chirila AM et al (2015) Three-dimensional neural spheroid culture: an in vitro model for cortical studies. Tissue Eng Part C Methods 21:1274–1283. https://doi.org/10.1089/ten.TEC.2015.0135
Dos Reis RS, Sant S, Ayyavoo V (2023) Three-dimensional human brain organoids to model HIV-1 neuropathogenesis. Methods Mol Biol 2610:167–178. https://doi.org/10.1007/978-1-0716-2895-9_14
dos Reis RS, Sant S, Keeney H et al (2020) Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Sci Rep 10:15209. https://doi.org/10.1038/s41598-020-72214-0
Douaud G, Lee S, Alfaro-Almagro F et al (2022) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604:697–707. https://doi.org/10.1038/s41586-022-04569-5
Enting RH, Prins JM, Jurriaans S et al (2001) Concentrations of human immunodeficiency virus type 1 (HIV-1) RNA in cerebrospinal fluid after antiretroviral treatment initiated during primary HIV-1 infection. Clin Infect Dis 32:1095–1099. https://doi.org/10.1086/319602
Eura N, Matsui TK, Luginbühl J et al (2020) Brainstem organoids from human pluripotent stem cells. Front Neurosci 14:538. https://doi.org/10.3389/fnins.2020.00538
Ferenczy MW, Johnson KR, Marshall LJ et al (2013) Differentiation of human fetal multipotential neural progenitor cells to astrocytes reveals susceptibility factors for JC virus. J Virol 87:6221–6231. https://doi.org/10.1128/JVI.00396-13
Ferenczy MW, Marshall LJ, Nelson CDS et al (2012) Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 25:471–506. https://doi.org/10.1128/CMR.05031-11
Gabriel E, Gopalakrishnan J (2017) Generation of iPSC-derived human brain organoids to model early neurodevelopmental disorders. JoVE 55372. https://doi.org/10.3791/55372
Gabriel E, Ramani A, Karow U et al (2017) Recent zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 20:397-406.e5. https://doi.org/10.1016/j.stem.2016.12.005
Garcez PP, Loiola EC, Madeiro da Costa R et al (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352:816–818. https://doi.org/10.1126/science.aaf6116
Garcia-Mesa Y, Jay TR, Checkley MA et al (2017) Immortalization of primary microglia: a new platform to study HIV regulation in the central nervous system. J Neurovirol 23:47–66. https://doi.org/10.1007/s13365-016-0499-3
Gebhardt BM, Halford WP (2005) Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J 2:67. https://doi.org/10.1186/1743-422X-2-67
González-Scarano F, Martín-García J (2005) The neuropathogenesis of AIDS. Nat Rev Immunol 5:69–81. https://doi.org/10.1038/nri1527
Gumbs SBH, Berdenis van Berlekom A, Kübler R et al (2022) Characterization of HIV-1 infection in microglia-containing human cerebral organoids. Viruses 14:829. https://doi.org/10.3390/v14040829
Gussow AM, Giordani NV, Tran RK et al (2006) Tissue-specific splicing of the herpes simplex virus type 1 latency-associated transcript (LAT) intron in LAT transgenic mice. J Virol 80:9414–9423. https://doi.org/10.1128/JVI.00530-06
Haenseler W, Sansom SN, Buchrieser J et al (2017) A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Reports 8:1727–1742. https://doi.org/10.1016/j.stemcr.2017.05.017
Hong YJ, Lee S been, Choi J et al (2022) A simple method for generating cerebral organoids from human pluripotent stem cells. IJSC 15:95–103. https://doi.org/10.15283/ijsc21195
Hou Y, Li C, Yoon C et al (2022) Enhanced replication of SARS-CoV-2 omicron BA.2 in human forebrain and midbrain organoids. Sig Transduct Target Ther 7:381. https://doi.org/10.1038/s41392-022-01241-2
Huang WK, Wong SZH, Pather SR et al (2021) Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell 28:1657-1670.e10. https://doi.org/10.1016/j.stem.2021.04.006
Huangui Xiong HT (2013) Astrocyte Dysfunctions and HIV-1 Neurotoxicity. J AIDS Clin Res 04. https://doi.org/10.4172/2155-6113.1000255
Jacob F, Pather SR, Huang W-K et al (2020) Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell 27:937-950.e9. https://doi.org/10.1016/j.stem.2020.09.016
Jo J, Xiao Y, Sun AX et al (2016) Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19:248–257. https://doi.org/10.1016/j.stem.2016.07.005
Karagiannis P, Kim S-I (2021) iPSC-derived natural killer cells for cancer immunotherapy. Mol Cells 44:541–548. https://doi.org/10.14348/molcells.2021.0078
Karzbrun E, Kshirsagar A, Cohen SR et al (2018) Human brain organoids on a chip reveal the physics of folding. Nat Phys 14:515–522. https://doi.org/10.1038/s41567-018-0046-7
Kim J-Y, Fluri DA, Marchan R et al (2015) 3D spherical microtissues and microfluidic technology for multi-tissue experiments and analysis. J Biotechnol 205:24–35. https://doi.org/10.1016/j.jbiotec.2015.01.003
Kimberlin DW (2004) Neonatal herpes simplex infection. Clin Microbiol Rev 17:1–13. https://doi.org/10.1128/CMR.17.1.1-13.2004
King CA, Li X, Barbachano-Guerrero A, Bhaduri-McIntosh S (2015) STAT3 regulates lytic activation of kaposi’s sarcoma-associated herpesvirus. J Virol 89:11347–11355. https://doi.org/10.1128/JVI.02008-15
Kondo Y, Windrem MS, Zou L et al (2014) Human glial chimeric mice reveal astrocytic dependence of JC virus infection. J Clin Invest 124:5323–5336. https://doi.org/10.1172/JCI76629
Kong W, Montano M, Corley MJ et al (2022) Neuropilin-1 Mediates SARS-CoV-2 infection of astrocytes in brain organoids, inducing inflammation leading to dysfunction and death of neurons. mBio 13:e0230822. https://doi.org/10.1128/mbio.02308-22
Kovalevich J, Langford D (2012) Neuronal toxicity in HIV CNS disease. Future Virol 7:687–698. https://doi.org/10.2217/fvl.12.57
Krenn V, Bosone C, Burkard TR et al (2021) Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell Stem Cell 28:1362-1379.e7. https://doi.org/10.1016/j.stem.2021.03.004
Lafaille FG, Pessach IM, Zhang S-Y et al (2012) Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491:769–773. https://doi.org/10.1038/nature11583
Lancaster MA, Knoblich JA (2014) Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9:2329–2340. https://doi.org/10.1038/nprot.2014.158
Lancaster MA, Renner M, Martin CA et al (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. https://doi.org/10.1038/nature12517
Li Puma DD, Piacentini R, Leone L et al (2019) Herpes simplex virus type-1 infection impairs adult hippocampal neurogenesis via amyloid-β protein accumulation. Stem Cells 37:1467–1480. https://doi.org/10.1002/stem.3072
Li Z, Xu J, Lang Y et al (2020) JMX0207, a niclosamide derivative with improved pharmacokinetics, suppresses zika virus infection both in vitro and in vivo. ACS Infect Dis 6:2616–2628. https://doi.org/10.1021/acsinfecdis.0c00217
Li Z, Xu J, Lang Y et al (2022) In vitro and in vivo characterization of erythrosin B and derivatives against Zika virus. Acta Pharmaceutica Sinica B 12:1662–1670. https://doi.org/10.1016/j.apsb.2021.10.017
Linard M, Letenneur L, Garrigue I et al (2020) Interaction between APOE4 and herpes simplex virus type 1 in Alzheimer’s disease. Alzheimer’s Dement 16:200–208. https://doi.org/10.1002/alz.12008
Lindenbach BD, Rice CM (2003) Molecular biology of flaviviruses. In: Advances in Virus Research. Elsevier, pp 23–61
Liu J, Li Y, Liu Q et al (2021) SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov 7:17. https://doi.org/10.1038/s41421-021-00249-2
Liu L, Chen Z, Zhang X et al (2019) Protection of ZIKV infection-induced neuropathy by abrogation of acute antiviral response in human neural progenitors. Cell Death Differ 26:2607–2621. https://doi.org/10.1038/s41418-019-0324-7
Liu P, Chen M, Liu Y et al (2018) CRISPR-based chromatin remodeling of the endogenous Oct4 or Sox2 locus enables reprogramming to pluripotency. Cell Stem Cell 22:252-261.e4. https://doi.org/10.1016/j.stem.2017.12.001
Liu R, Meng X, Yu X et al (2022) From 2D to 3D co-culture systems: a review of co-culture models to study the neural cells interaction. IJMS 23:13116. https://doi.org/10.3390/ijms232113116
Lyadova I, Vasiliev A (2022) Macrophages derived from pluripotent stem cells: prospective applications and research gaps. Cell Biosci 12:96. https://doi.org/10.1186/s13578-022-00824-4
Major EO, Miller AE, Mourrain P et al (1985) Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci USA 82:1257–1261. https://doi.org/10.1073/pnas.82.4.1257
Major EO, Vacante DA (1989) Human fetal astrocytes in culture support the growth of the neurotropic human polyomavirus. JCV: J Neuropathol Exper Neurol 48:425–436. https://doi.org/10.1097/00005072-198907000-00004
Mallard J, Williams KC (2018) Animal models of HIV-associated disease of the central nervous system. In: Handbook of Clinical Neurology. Elsevier, pp 41–53
Mandl C, Walker DL, Frisque RJ (1987) Derivation and characterization of POJ cells, transformed human fetal glial cells that retain their permissivity for JC virus. J Virol 61:755–763. https://doi.org/10.1128/jvi.61.3.755-763.1987
Mansour AA, Gonçalves JT, Bloyd CW et al (2018) An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 36:432–441. https://doi.org/10.1038/nbt.4127
Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol 77:683. https://doi.org/10.1001/jamaneurol.2020.1127
Marquez L, Levy ML, Munoz FM, Palazzi DL (2011) A report of three cases and review of intrauterine herpes simplex virus infection. Pediatric Infectious Disease Journal 30:153–157. https://doi.org/10.1097/INF.0b013e3181f55a5c
Matsumoto R, Suga H, Aoi T et al (2020) Congenital pituitary hypoplasia model demonstrates hypothalamic OTX2 regulation of pituitary progenitor cells. J Clin Invest 130:641–654. https://doi.org/10.1172/JCI127378
Mazzara PG, Criscuolo E, Rasponi M et al (2022) A human stem cell-derived neurosensory–epithelial circuitry on a chip to model herpes simplex virus reactivation. Biomedicines 10:2068. https://doi.org/10.3390/biomedicines10092068
McMahon CL, Staples H, Gazi M et al (2021) SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Reports 16:1156–1164. https://doi.org/10.1016/j.stemcr.2021.01.016
Mesci P, de Souza JS, Martin-Sancho L et al (2022) SARS-CoV-2 infects human brain organoids causing cell death and loss of synapses that can be rescued by treatment with Sofosbuvir. PLoS Biol 20:e3001845. https://doi.org/10.1371/journal.pbio.3001845
Messacar K, Fischer M, Dominguez SR et al (2018) Encephalitis in US children. Infect Dis Clin North Am 32:145–162. https://doi.org/10.1016/j.idc.2017.10.007
Messam CA, Hou J, Gronostajski RM, Major EO (2003) Lineage pathway of human brain progenitor cells identified by JC virus susceptability. Ann Neurol 53:636–646. https://doi.org/10.1002/ana.10523
Miller F, Afonso PV, Gessain A, Ceccaldi P-E (2012) Blood-Brain Barrier and Retroviral Infections Virulence 3:222–229. https://doi.org/10.4161/viru.19697
Miskin DP, Koralnik IJ (2015) Novel syndromes associated with JC virus infection of neurons and meningeal cells: no longer a gray area. Curr Opin Neurol 28:288–294. https://doi.org/10.1097/WCO.0000000000000201
Miura Y, Li MY, Revah O et al (2022) Engineering brain assembloids to interrogate human neural circuits. Nat Protoc 17:15–35. https://doi.org/10.1038/s41596-021-00632-z
Miyamura T, Yoshiike K, Takemoto KK (1980) Characterization of JC papovavirus adapted to growth in human embryonic kidney cells. J Virol 35:498–504. https://doi.org/10.1128/jvi.35.2.498-504.1980
Mlakar J, Korva M, Tul N et al (2016) Zika virus associated with microcephaly. N Engl J Med 374:951–958. https://doi.org/10.1056/NEJMoa1600651
Ng J-H, Sun A, Je HS, Tan E-K (2023) Unravelling pathophysiology of neurological and psychiatric complications of COVID-19 using brain organoids. Neuroscientist 29:30–40. https://doi.org/10.1177/10738584211015136
Nilsson P, Iwata N, Muramatsu S et al (2010) Gene therapy in Alzheimer’s disease - potential for disease modification. J Cell Mol Med 14:741–757. https://doi.org/10.1111/j.1582-4934.2010.01038.x
O’Brien BS, Mokry RL, Schumacher ML et al (2022) Downregulation of neurodevelopmental gene expression in iPSC-derived cerebral organoids upon infection by human cytomegalovirus. iScience 25:104098. https://doi.org/10.1016/j.isci.2022.104098
Ormel PR, Vieira de Sá R, van Bodegraven EJ et al (2018) Microglia innately develop within cerebral organoids. Nat Commun 9:4167. https://doi.org/10.1038/s41467-018-06684-2
Padgett BL, Walker DL, ZuRhein GM et al (1971) Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1:1257–1260. https://doi.org/10.1016/s0140-6736(71)91777-6
Pamies D, Barreras P, Block K et al (2017) A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. ALTEX 34:362–376. https://doi.org/10.14573/altex.1609122
Pellegrini L, Albecka A, Mallery DL et al (2020) SARS-CoV-2 Infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell 27:951-961.e5. https://doi.org/10.1016/j.stem.2020.10.001
Pham MT, Pollock KM, Rose MD et al (2018) Generation of human vascularized brain organoids. NeuroReport 29:588–593. https://doi.org/10.1097/WNR.0000000000001014
Pichler M, Staffler A, Bonometti N et al (2015) Premature newborns with fatal intrauterine herpes simplex virus-1 infection: first report of twins and review of the literature. J Eur Acad Dermatol Venereol 29:1216–1220. https://doi.org/10.1111/jdv.12583
Qian X, Nguyen HN, Song MM et al (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254. https://doi.org/10.1016/j.cell.2016.04.032
Qiao H, Guo M, Shang J et al (2020) Herpes simplex virus type 1 infection leads to neurodevelopmental disorder-associated neuropathological changes. PLoS Pathog 16:e1008899. https://doi.org/10.1371/journal.ppat.1008899
Qiao H, Zhao W, Guo M et al (2022) Cerebral organoids for modeling of HSV-1-induced-amyloid β associated neuropathology and phenotypic rescue. IJMS 23:5981. https://doi.org/10.3390/ijms23115981
Rai MA, Hammonds J, Pujato M et al (2020) Comparative analysis of human microglial models for studies of HIV replication and pathogenesis. Retrovirology 17:35. https://doi.org/10.1186/s12977-020-00544-y
Raja WK, Mungenast AE, Lin YT et al (2016) Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PLoS One 11:e0161969. https://doi.org/10.1371/journal.pone.0161969
Ramani A, Müller L, Ostermann PN et al (2020) SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J 39:e106230. https://doi.org/10.15252/embj.2020106230
Rawat P, Spector SA (2017) Development and characterization of a human microglia cell model of HIV-1 infection. J Neurovirol 23:33–46. https://doi.org/10.1007/s13365-016-0472-1
Reynolds BA, Tetzlaff W, Weiss S (1992) A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci 12:4565–4574. https://doi.org/10.1523/JNEUROSCI.12-11-04565.1992
Salick MR, Wells MF, Eggan K, Kaykas A (2017) Modelling Zika virus infection of the developing human brain in vitro using stem cell derived cerebral organoids. J Vis Exp 56404. https://doi.org/10.3791/56404
Samudyata OAO, Malwade S et al (2022) SARS-CoV-2 promotes microglial synapse elimination in human brain organoids. Mol Psychiatry 27:3939–3950. https://doi.org/10.1038/s41380-022-01786-2
Schaumburg C, O’Hara BA, Lane TE, Atwood WJ (2008) Human embryonic stem cell-derived oligodendrocyte progenitor cells express the serotonin receptor and are susceptible to JC virus infection. J Virol 82:8896–8899. https://doi.org/10.1128/JVI.00406-08
Sison SL, O’Brien BS, Johnson AJ et al (2019) Human cytomegalovirus disruption of calcium signaling in neural progenitor cells and organoids. J Virol 93:e00954-e1019. https://doi.org/10.1128/JVI.00954-19
Soares R, Ribeiro FF, Lourenço DM et al (2021) The neurosphere assay: an effective. Neural Regen Res 16:2229–2231. https://doi.org/10.4103/1673-5374.310678
Song E, Zhang C, Israelow B et al (2021) Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exper Med 218:e20202135. https://doi.org/10.1084/jem.20202135
Straface G, Selmin A, Zanardo V et al (2012) Herpes simplex virus infection in pregnancy. Infect Dis Obs Gynecol 1–6. https://doi.org/10.1155/2012/385697
Sun G, Chiuppesi F, Chen X et al (2020) Modeling human cytomegalovirus-induced microcephaly in human iPSC-derived brain organoids. Cell Rep Med 1:100002. https://doi.org/10.1016/j.xcrm.2020.100002
Sun XY, Ju XC, Li Y et al (2022) Generation of vascularized brain organoids to study neurovascular interactions. Elife 11. https://doi.org/10.7554/eLife.76707
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. https://doi.org/10.1016/j.cell.2007.11.019
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. https://doi.org/10.1016/j.cell.2006.07.024
Thompson RL, Shieh MT, Sawtell NM (2003) Analysis of herpes simplex virus ICP0 promoter function in sensory neurons during acute infection, establishment of latency, and reactivation in vivo. J Virol 77:12319–12330. https://doi.org/10.1128/JVI.77.22.12319-12330.2003
Tiwari SK, Wang S, Smith D et al (2021) Revealing tissue-specific SARS-CoV-2 infection and host responses using human stem cell-derived lung and cerebral organoids. Stem Cell Reports 16:437–445. https://doi.org/10.1016/j.stemcr.2021.02.005
Vahsen BF, Gray E, Candalija A et al (2022) Human iPSC co-culture model to investigate the interaction between microglia and motor neurons. Sci Rep 12:12606. https://doi.org/10.1038/s41598-022-16896-8
Volpato V, Webber C (2020) Addressing variability in iPSC-derived models of human disease: guidelines to promote reproducibility. Dis Model Mech 13:dmm042317. https://doi.org/10.1242/dmm.042317
Wallet C, De Rovere M, Van Assche J et al (2019) Microglial cells: the main HIV-1 reservoir in the brain. Front Cell Infect Microbiol 9:362. https://doi.org/10.3389/fcimb.2019.00362
Wang C, Zhang M, Garcia G et al (2021) ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell 28:331-342.e5. https://doi.org/10.1016/j.stem.2020.12.018
Weissert R (2011) Progressive multifocal leukoencephalopathy. J Neuroimmunol 231:73–77. https://doi.org/10.1016/j.jneuroim.2010.09.021
Wells MF, Salick MR, Wiskow O et al (2016) Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from zika virus infection. Cell Stem Cell 19:703–708. https://doi.org/10.1016/j.stem.2016.11.011
Wiley CA, Achim CL, Christopherson C et al (1999) HIV mediates a productive infection of the brain. AIDS 13:2055–2059. https://doi.org/10.1097/00002030-199910220-00007
Xiang Y, Cakir B, Park IH (2020) Generation of regionally specified human brain organoids resembling thalamus development. STAR Protoc 1. https://doi.org/10.1016/j.xpro.2019.100001
Xiang Y, Tanaka Y, Cakir B et al (2019) hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell 24:487-497.e7. https://doi.org/10.1016/j.stem.2018.12.015
Xu M, Lee EM, Wen Z et al (2016) Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 22:1101–1107. https://doi.org/10.1038/nm.4184
Xu Y-P, Qiu Y, Zhang B et al (2019) Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res 29:265–273. https://doi.org/10.1038/s41422-019-0152-9
Yi SA, Nam KH, Yun J et al (2020) Infection of brain organoids and 2D cortical neurons with SARS-CoV-2 pseudovirus. Viruses 12:1004. https://doi.org/10.3390/v12091004
Zayyad Z, Spudich S (2015) Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr HIV/AIDS Rep 12:16–24. https://doi.org/10.1007/s11904-014-0255-3
Zhang B, He Y, Xu Y et al (2018) Differential antiviral immunity to Japanese encephalitis virus in developing cortical organoids. Cell Death Dis 9:719. https://doi.org/10.1038/s41419-018-0763-y
Zhang B-Z, Chu H, Han S et al (2020) SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res 30:928–931. https://doi.org/10.1038/s41422-020-0390-x
Zhao Z, Chen X, Dowbaj AM et al (2022) Organoids Nat Rev Methods Primers 2:94. https://doi.org/10.1038/s43586-022-00174-y
Zheng W, Benner EM, Bloom DC et al (2022) Variations in aspects of neural precursor cell neurogenesis in a human model of HSV-1 infection. Organogenesis 18:2055354. https://doi.org/10.1080/15476278.2022.2055354
Zheng W, Klammer AM, Naciri JN et al (2020) Patterns of herpes simplex virus 1 infection in neural progenitor cells. J Virol 94:e00994-e1020. https://doi.org/10.1128/JVI.00994-20
Zhou S, Szczesna K, Ochalek A et al (2016) Neurosphere based differentiation of human iPSC improves astrocyte differentiation. Stem Cells Int 2016:4937689. https://doi.org/10.1155/2016/4937689
Zhou T, Tan L, Cederquist GY et al (2017) High-content screening in hPSC-neural progenitors identifies drug candidates that inhibit zika virus infection in fetal-like organoids and adult brain. Cell Stem Cell 21:274-283.e5. https://doi.org/10.1016/j.stem.2017.06.017
Zimmer B, Ewaleifoh O, Harschnitz O et al (2018) Human iPSC-derived trigeminal neurons lack constitutive TLR3-dependent immunity that protects cortical neurons from HSV-1 infection. Proc Natl Acad Sci USA 115. https://doi.org/10.1073/pnas.1809853115
Acknowledgements
The authors wish to thank past and present members of the Department of Microbiology, Immunology and Inflammation and the Center for Neurovirology and Gene Editing for sharing reagents and ideas.
Funding
This work was supported by grants (NIH-R01 DA052284-01A1; 2 R01 MH110360-06) awarded by the NIH to IKS. This study utilized services offered by core facilities of the Comprehensive NeuroHIV Center (CNHC NIMH Grant Number P30MH092177-11).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swingler, M., Donadoni, M., Bellizzi, A. et al. iPSC-derived three-dimensional brain organoid models and neurotropic viral infections. J. Neurovirol. 29, 121–134 (2023). https://doi.org/10.1007/s13365-023-01133-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-023-01133-3