Abstract
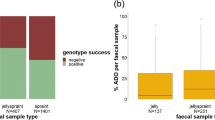
Non-invasive genetics is a powerful tool in wildlife research and monitoring, especially when dealing with elusive and rare species such as the Eurasian otter (Lutra lutra). Nevertheless, otter DNA obtained from scats and anal secretions appears to be exposed to very quick degradation processes, and the success rate in DNA amplification is lower than in other carnivores. We collected 191 samples from April to September 2011 along the river Sangro basin (Italy) which was recently re-colonized by the Eurasian otter. Using two sets of microsatellite loci (six Lut and seven OT loci), we investigated the influence of sample type and age, collection time, storage time, temperature and humidity on genotyping success and amplification success. We also tested the efficacy of different DNA extraction kits and storage buffer mediums. Finally, we compared amplification success rate, allelic dropout and false allele rates for each locus. We obtained a mean amplification success rate of 79.0 % and a genotyping success rate of 35.1 %. Fresh pure jellies yielded the highest amplification success and genotyping rate. Six microsatellite loci should be theoretically sufficient to distinguish the individual unrelated otters (PID = 0.001), while 13 loci were needed to distinguish sibling otters (PIDsibs = 0.002) in our population. We identified 11 otters, and molecular sexing ascertained the presence of five males, four females and two uncertain individuals. Generalized linear models highlighted a significant influence of sample type and age, temperature and humidity both on genotyping and amplification success.


Similar content being viewed by others
References
Arandjelovic M, Head J, Rabanal LI, Schubert G, Mettke E, Boesch C, Robbins M, Vigilant L (2011) Non-invasive genetic monitoring of wild central chimpanzees. PLoS ONE 6(3)
Arrendal J, Vilà C, Björklund M (2007) Reliability of non invasive genetic census of otters compared to field censuses. Conserv Genet 8:1097–1107
Bjǒrklund M (2010) A method for adjusting allele frequencies in the case of microsatellite allele dropout. Molec Ecol Notes 5:676–679
Bonesi L, Hale M, Macdonald DW (2012) Lessons from the use of non-invasive genetic sampling as a way to estimate Eurasian otter population size and sex ratio. Acta Theriol 58(2):157–168
Bonin A, Bellamain E, Eidesen PB et al (2004) How to track and assess genotyping errors in population genetic studies. Mol Ecol 13:3261–3273
Broquet T, Ménard N, Petit E (2007) Noninvasive population genetics: a review of sample sources, diet, fragment length and microsatellite motif effect on amplification success and genotyping error rates. Conserv Genet 8:249–260
Brzeski KF, Szykman Gunther M, Black JM (2013) Evaluating river otter demography using noninvasive genetic methods. J Wildlife Manage 77(8):1523–1531
Buchan JC, Archie EA, Van Horn RC, Moss CJ, Alberts SC (2005) Locus effects and sources of error in noninvasive genotyping. Mol Ecol 5:680–683
Caniglia R, Fabbri E, Cubaynes S, Gimenez O, Lebreton JD, Rande E (2012) An improved procedure to estimate wolf abundance using non-invasive genetic sampling and capture-recapture mixture models. Conserv Genet 13:53–64
Cassola F (1986) La lontra in Italia. Censimento, distribuzione e problemi di conservazione di una specie minacciata. In: The Otter in Italy. Survey, distribution e conservation of an endangered species. WWF Italia, Serie Atti e Studi n.5, Roma, 135 pp
Chanin P (1985) The natural history of otters. Facts on File Inc., New York
Cohen TM, Narkiss T, Dolev A, Ben-Ari Y, Kronfeld-Schor N, Guter A, Saltz D, Bar-Gal GK (2013) Genetic diversity of the Eurasian otter (Lutra lutra) population in Israel
Conroy JWH, Chanin PF (2000) The status of the Eurasian otter (Lutra lutra) in Europe—a review. In: Conroy JWH, Yoxon P, Gutleb AC (eds.) Proceedings of the first otter toxicology conference, Isle of Skye. September 2000. Journal of the International Otter Survival Fund No 1:7–28
Coxon K, Chanin P, Dallas J, Sykes T (1999) The use of DNA fingerprinting to study population dynamics of otters (Lutra lutra) in Southern Britain: a feasibility study. R&D Technical Report W202, Environment Agency, Bristol, United Kingdom
Dallas JF, Piertney SB (1998) Microsatellite primers for Eurasian otter. Mol Ecol 7:1247–1251
Dallas JF, Marshall F, Piertney SB, Bacon PJ, Racey PA (2002) Spatially restricted gene flow and reduced microsatellite polymorphism in the Eurasian otter Lutra lutra in Britain. Conserv Genet 3:15–29
Dallas JF, Coxon KE, Sykes T, Chanin PRF, Marshall F, Carss DN, Bacon PJ, Piertney SB, Racey PA (2003) Similar estimates of population genetic composition and sex ratio derived from carcasses and faeces of Eurasian otter Lutra lutra. Mol Ecol 12:275–282
Farrell LE, Roman J, Sunquist ME (2000) Dietary separation of sympatric carnivores identified by molecular analysis of scats. Mol Ecol 9:1583–1590
Ferrando A, Lecis R, Domingo-Roura X, Ponsà M (2008) Genetic diversity and individual identification of reintroduces otters (Lutra lutra) in north-eastern Spain by DNA genotyping of spraints. Conserv Genet 9:129–139
Fike JA, Serfass TL, Beheler AS, Rhodes OE Jr (2004) Genotyping error rates associated with alternative sources of DNA for the north American river otter. IUCN Otter Spec Group Bull 21A
Frantz AC, Pope LC, Carpenter PJ, Roper TJ, Wilson GJ, Delahay RJ, Burke T (2003) Reliable microsatellite genotyping of the Eurasian badger (Meles meles) using faecal DNA
Frantzen MAJ, Silk JB, Fergusen JWH, Wayne RK, Kohn MH (1998) Empirical evaluation of preservation methods for faecal DNA. Mol Ecol 7:1423–1428
Gorman ML, Trowbridge BJ (1989) The role of odor in the social lives of carnivores. In: Git-tleman JL (ed) Carnivore behavior, ecology, and evolution. Cornell University, Ithaca, pp 57–88
Guertin DA, Ben-David M, Harestad AS, Elliott JE (2012) Fecal genotyping reveals demographic variation in river otters inhabiting a contaminated environment. J Wildlife Manage
Hájková P, Zemanova B, Bryja J, Hájek B, Roche K, Tkadlec E, Zima J (2006) Factors affecting success of PCR amplification of microsatellite loci from otter faeces. Mol Ecol Notes 6:559–562
Hájková P, Zemanová B, Roche K, Hájek B (2009) An evaluation of field and non invasive genetic methods for estimating Eurasian otter population size. Conserv Genet 10:1667–1681
Holleley CE, Geerts PG (2009) Multiplex Manager 1.0: a crossplatform computer program that plans and optimizes multiplex PCR. BioTechniques 46(7):511–517
Huang CC, Hsu YC, Lee LL, Li SH (2005) Isolation and characterization of tetramicrosatellite DNA markers in the Eurasian otter (Lutra lutra). Mol Ecol 5:314–316
Hung CM, Li SH, Lee LL (2004) Faecal DNA typing to determine the abundance and spatial organisation of otters (Lutra lutra) along two stream systems in Kinmen. Anim Conserv 7:301–311
Jansman HAH, Chanin PRF, Dallas JF (2001) Monitoring otter populations by DNA typing of spraints. IUCN Otter Spec Bull 18:12–19
Kalz B, Jewgenow K, Fickel J (2006) Structure of an otter (Lutra lutra) population in Germany—results of DNA and hormone analyses from faecal samples. Mamm Biol 71(6):321–335
Koelewijn HP, Pérez-Haro M, Jansman HAH, Boerwinkel MC, Bovenschen J, Lammertsma DR, Niewold FJJ, Kuiters AT (2010) The reintroduction of the Eurasian otter (Lutra lutra) into the Netherlands: hidden life revealed by noninvasive genetic monitoring. Conserv Genet 11:601–614
Kruuk H (2006) Otters ecology, behaviour and conservation, 2nd edn. Oxford University, Oxford
Lampa S, Gruber B, Henle K, Hoehn M (2008) An optimization approach to increase DNA amplification success of otter faeces. Conserv Genet 9:201–210
Lanszki J, Hidas A, Szentes K, Révay T, Lehoczky I, Weiss S (2008) Relative spraint density and genetic structure of otter (Lutra lutra) along Drava River in Hungary. Mamm Biol 73:40–47
Lerone L, Mengoni C, Randi E, Carpaneto GM, and Loy A (2011) Non-invasive genetic sampling of Eurasian otter in its Italian northern range. IUCN-XIth International Otter Colloquium- Otters in a warming world (Pavia, Italy, 2011). Hystrix It J Mamm (n.s.), Suppl. 2011: 109
Loy A, Bucci L, Carranza ML, De Castro G, Di Marzio P, Reggiani G (2004) Survey and habitat evaluation for a peripherical population of the Eurasian otter in Italy. IUCN Otter Spec. Group Bull. 21A
Loy A, Boitani L, Bonesi L, Canu A, Di Croce A, Fiorentino PL, Genovesi P, Mattei L, Panzacchi M, Prigioni C, Randi E, Reggiani G (2010) The Italian action plan for the endangered Eurasian otter Lutra lutra. Hystrix It J Mamm (ns) 21(1):19–33
Macdonald SM, Mason CF (1983) The Otter Lutra lutra in Southern Italy. Biol Conserv 25(2):95–101
Macdonald SM, Mason CF (1994) Status and conservation need of the otter (Lutra lutra) in the western Paleartic. Nat Environment 67:1–54, Council of Europe Press
McKelvey KS, Schwartz MK (2004) Genetic errors associated with population estimation using non-invasive molecular tagging: problems and new solutions. J Wildlife Manage 68:439–448
Mowry RA, Gompper ME, Eggert LS (2011) River otter population size estimation using noninvasive latrine surveys. J Wildlife Manage 75(7):1625–1636
Mucci N, Randi E (2007) Sex identification of Eurasian otter (Lutra lutra) non-invasive DNA samples using ZFX/ZFY sequences. Conserv Genet 8:1479–1482
Mucci N, Arrendal J, Ansorge H, Bailey M, Bodner M, Delibes M, Ferrando A, Fournier P, Fournier C, Godoy JA, Hajkova P, Hauer S, Heggberget TM, Heidecke D, Kirjavainen H, Krueger HH, Kvaloy K, Lafontaine L, Lanszki J, Lemarchand C, Liukko UM, Loeschcke V, Ludwig G, Madsen AB, Mercier L, Ozolins J, Paunovic M, Pertoldi C, Piriz A, Prigioni C, Santos-Reis M, Sales Luis T, Stjernberg T, Schmid H, Suchentrunk F, Teubner J, Tornberg R, Zinke O, Randi E (2010) Genetic diversity and landscape genetic structure of otter (Lutra lutra) populations in Europe. Conserv Genet 11(2):583–599
Murphy MA, Waits LP, Kendall KC (2003) The influence of diet on faecal DNA amplification and sex identification in brown bears (Ursus arctos). Mol Ecol 12:2261–2265
Murphy MA, Kendall KC, Robinson A, Waits LP (2007) The impact of time and field conditions on brown bear (Ursus arctos) faecal DNA amplification. Conserv Genet 8:1219–1224
Nsubuga AM, Robbins MM, Roeder AD, Morin PA, Boesch C, Vigilant L (2004) Factors affecting the amount of genomic DNA extracted from ape faeces and the identification of an improved sample storage method. Mol Ecol 13:2089–2094
Panzacchi M, Genovesi P, Loy A (2010) Piano d’azione nazionale per la conservazione della lontra (Lutra lutra). Min. Ambiente - ISPRA
Paetkau D (2003) An empirical exploration of data quality in DNA-based population inventories. Mol Ecol 12:1375–1387
Peakall R, Smouse PE (2006) GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Ecology Notes 6:288–295
Pennell MW, Stansbury CR, Waits LP, Miller CR (2013) Capwire: an R package for estimating population census size from non-invasive genetic sampling. Mol Ecol Resour 13:154–157
Prigioni C, Remonti L, Balestrieri A, Sgrosso S, Priore G, Mucci N, Randi E (2006) Estimation of European otter (Lutra lutra) population size by fecal DNA typing in Southern Italy. J Mamm 87(5):855–858
Prigioni C, Balestrieri A, Remonti L (2007) Decline and recovery in otter Lutra lutra populations in Italy. Mamm Rev 37:71–79
Quaglietta L, Fonseca VC, Hájková P, Mira A, Boitani L (2013) Fine-scale population genetic structure and short-range sex-biased dispersal in a solitary carnivore, Lutra lutra. J Mammal 94(3):561–571
Randi E, Davoli F, Pierpaoli M, Pertoldi C, Madsen AB, Loeschcke V (2003) Genetic structure in otter (Lutra lutra) populations in Europe: implications for conservation. Anim Conserv 6:93–100
Reuther C, Dolch D, Green R, Jahrl J, Jefferies D, Krekemeyer A, Kucerova M, Madesn AB, Romanowsky J, Roche K, Ruiz-Olmo J, Teubner J, Trinidade A (2000) Surveying and monitoring distribution and population trends of the Eurasian otter (Lutra lutra): guidelines and evaluation of the standard method for surveys as recommended by the European Section of the IUCN/SSC Otter Specialist Group. Habitat 12
Rondinini C, Battistoni A, Peronace V, Teofili C (2013) Lista Rossa IUCN dei Vertebrati Italiani. Comitato Italiano IUCN e Ministero dell’Ambiente e dela Tutela del Territorio e del Mare, Roma
Ruiz-Olmo J, Loy A, Cianfrani C, Yoxon P, Yoxon G, de Silva PK, Roos A, Bisther M, Hájková P, Zemanová B (2008) Lutra lutra. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.1. www.iucnredlist.org. Accessed 30 May 2014
Silveira Trinca C, Fernandes Jaeger C, Eizirik E (2013) Molecular ecology of the Neotropical otter (Lontra longicaudis): non-invasive sampling yields insights into local population dynamics. Biol J Linn Soc 109:932–948
Spagnesi M, Toso S, De Marinis AM (eds) (2000) I Mammiferi d’Italia, Istituto Nazionale per la Fauna Selvatica. Ozzano Emilia, Italy
Spiering PA, Gunther MS, Wildt DE, Somers MJ, Maldonado JE (2009) Sampling error in non-invasive genetic analyses of an endangered social carnivore. Conserv Genet 10:2005–2007
Valiere N (2002) GIMLET: a computer program for analysing genetic individual identification data. Mol Ecol Notes 2:377–379
Waits LP, Paetkau D (2005) Noninvasive genetic sampling for wildlife biologists: a review of applications and recommendations for accurate data collection. J Wildlife Manage 69(4):1419–1433
Acknowledgments
We thank Prof. C. Pertoldi and the anonymous reviewer for their precious advices which greatly improved the manuscript. We also thank A. Brambilla, A. Leone, L. Nelli, A. Palladini, R. Caniglia, F. Davoli, E. Fabbri, F. Mattucci and N. Mucci for their suggestions and constant support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Cino Pertoldi
Rights and permissions
About this article
Cite this article
Lerone, L., Mengoni, C., Carpaneto, G.M. et al. Procedures to genotype problematic non-invasive otter (Lutra lutra) samples. Acta Theriol 59, 511–520 (2014). https://doi.org/10.1007/s13364-014-0195-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-014-0195-8