Abstract
The integration of the liquid sampling-atmospheric pressure glow discharge (LS-APGD) ion source with Orbitrap mass spectrometers has resulted in new opportunities in the field of isotope ratio mass spectrometry. In a field that has been dominated by thermal ionization mass spectrometry (TIMS) and inductively coupled plasma mass spectrometry (ICP-MS) on quadrupole and scanning-mode sector field analyzer platforms for highly accurate and precise measurements, the LS-APGD-Orbitrap system offers a benchtop instrument capable of meeting the rigorous International Target Values for measurement uncertainty for uranium (U). In order to benchmark the LS-APGD-Orbitrap, a series of U certified reference materials with increasing 235U isotopic composition were analyzed. By using U samples ranging in enrichment from 1 to 80%, the ability of the system to measure isotope ratios over a wide range is demonstrated. This analysis represents the first time that the LS-APGD-Orbitrap system has been used to analyze highly enriched U samples, allowing for the measurement of each of the U isotopes, including 234U and 236U-related species, which had not been achieved previously. Ultimately, the LS-APGD-Orbitrap system was able to measure CRM U-800 (assayed as 235U / 238U = 4.265622) as 4.266922, with a combined uncertainty, (uc), of 0.040%. These results are compared to those obtained using traditional elemental mass spectrometers including TIMS and ICP-MS-based instruments. The effectiveness of the LS-APGD-Orbitrap MS system for measuring U isotopes shows excellent promise in nuclear forensics, safeguards, and other nuclear weapon-based applications.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the advent of the atomic age, the fields of nuclear forensics and nuclear safeguards have relied heavily on mass spectrometry to measure isotopic abundances of uranium (U), plutonium (Pu), and related radionuclides with a high degree of accuracy and precision [1,2,3]. Due to the nature of these materials, special considerations must be made when performing the analysis, stemming from the need to ensure nuclear materials are appropriately accounted for in their respective uses (i.e., nuclear energy). It is vital, regarding U isotope ratio measurements, to be able to distinguish between 235U enrichment levels pertaining to civilian (0.02 – 0.05%), fuel (0.7 – 20%), and military (> 20%)-based applications [1,2,3]. In order to establish guidelines for safeguards verification, the International Atomic Energy Agency (IAEA) adopted the International Target Values (ITVs) for Measurement Uncertainties in Safeguarding Nuclear Materials (Table 1) to characterize the reliability of analytical techniques applied to industrial nuclear and fissile materials [4].
Current research and applications regarding atomic and isotope ratio mass spectrometry (IRMS) for the analysis of nuclear materials, has focused on thermal ionization mass spectrometry (TIMS) [5] and inductively coupled plasma-mass spectrometry (ICP-MS) instruments for solution-based sample analysis [6, 7], and laser ablation coupled ICP-MS (LA-ICP-MS) [8,9,10] and secondary ionization mass spectrometry (SIMS) [11] instruments for direct solids analysis. While these instruments are proven in their ability to perform isotope ratio measurements with a high degree of fidelity, they remain large and complex laboratory-based instruments, with long sample analysis times, particularly those associated with TIMS/SIMS. In comparison to TIMS and SIMS, ICP-MS instruments do have advantages in terms of high sample throughput, allowing the analysis of numerous samples as the ionization step does not have ultra-high vacuum sample introduction requirements. While there has been little change in the overall operation and instrumentation in ICP-MS, there have been advances in high efficiency sample introduction systems which have led to improved precision in isotope ratio measurements. For example, advanced spray chambers such as the stable sample introduction (SSI) dual quartz spray chamber (consisting of both a cyclonic and Scott-type spray chambers) housed within a Peltier-cooled apparatus or a desolvating nebulizer can improve sensitivity and precision as they homogenize the sample aerosol prior to introduction into the ICP, as well as reduce oxide formation [12,13,14]. Unfortunately, incremental advances seen in these techniques have not led to any significant advances with regard to field deployable isotope ratio analysis over the last few decades. In order to provide early diagnostic information and to expedite sample analysis required in nuclear forensic challenges, the development of a field deployable mass spectrometer is warranted. Of course, in order to realize such capabilities, both the ion source and mass analyzer components must be developed with regard to their fit-for-purpose qualities.
To this end, work at the Pacific Northwest National Laboratory and Clemson University has focused on developing the liquid sampling-atmospheric pressure glow discharge (LS-APGD) as a field deployable ion source for elemental and isotope ratio analysis [15,16,17,18]. The LS-APGD operates in a total consumption mode using a liquid electrolyte (sample) at a flow rate of 5 – 50 μL min−1, gas flow rate of < 1 L min−1, and power consumption of < 40 W, making it an attractive field deployable ion source. Over the course of its development the LS-APGD has been shown to be a versatile ion source. In addition to aqueous elemental/atomic samples, the LS-APGD is capable of determining organic and organometallic analytes while maintaining molecular structural information [19, 20]. Additionally, the LS-APGD has been used as a secondary ionization source for laser ablation samples and as an ambient desorption ion source for organic samples [21, 22]. While current research conducted by Marcus et al. has interfaced the LS-APGD with both 3D quadrupole traps and Orbitrap mass spectrometers, efforts for isotope ratio measurements have focused to using second-generation Orbitrap instruments due to their compact size potential and superior resolving power (m/Δm > 1,000,000). While Orbitrap mass spectrometers have been used extensively in the fields of proteomics and small-molecule analysis [23, 24], there is limited research looking at the ability of the Orbitrap to perform isotope ratio measurements. In related, very detailed work, Eiler and colleagues have used Orbitrap MS systems (GC Orbitrap and Q Exactive HF models) to evaluate the ability of the analyzer to measure stable isotopes (C, H, N, O, S) of organic molecules [25]. Use of the advanced quadrupole selector (AQS) and collisional activation methods to improve performance was demonstrated. In addition to electrospray ionization (ESI), conventional gas phase electron ionization (EI) was utilized successfully for isotope ratio measurements. Use of extended analysis times provided high levels of precision; single-°/°° in minutes and tenths-°/°° in tens of minutes.
While the LS-APGD work thus far has shown that Orbitrap instruments are capable of meeting the rigorous demands of the safeguards community for measurement uncertainty [17], there has been no direct comparison between the LS-APGD-Orbitrap system and current mass spectrometers used for atomic isotope ratio analysis. Of the various atomic systems currently available, TIMS remains the gold standard atomic mass spectrometer for U isotope ratio analysis [26,27,28,29]. More recently, multi-collector ICP-MS systems have distinguished themselves as the work horse instruments due to their ability to significantly decrease the analysis time while still meeting the rigorous requirements established in the ITVs [7, 13, 30]. In addition to these two specialized techniques, other commercial ICP-MS instruments, including single quadrupole (ICP-QMS) and scanning-mode magnetic sector (i.e. sector field, SF) instruments (ICP-SFMS) make up a significant market share in the atomic mass spectrometry community. In this research, we benchmark the isotope ratio measurement performance of the LS-APGD-Orbitrap system against commercial instruments including scanning SF and quadrupole-based ICP-MS systems and a multi-collector TIMS instrument. The results presented here include the analysis of highly enriched U samples as well as measuring an “unknown” sample. Ultimately, a sample containing 80% 235U was analyzed by the LS-APGD-Orbitrap system with a total measurement uncertainty of 0.04%, suggesting great promise for isotope ratio measurements.
Materials and Methods
Sample Preparation
In order to benchmark the LS-APGD-Orbitrap system against traditional atomic mass spectrometry methods for uranium isotope ratio analysis, a series of highly purified U oxide (U3O8) certified reference materials (CRM) were examined. These CRMs, U-010, U-100, U-500, and U-800 (New Brunswick Laboratory, Argonne, IL, USA), are primarily employed as quantitative and isotopic standards for performing U measurements. U-010, U-100, U-500, and U-800 were prepared by dissolving 100 mg in ~ 2 mL of 8 M HNO3 at 80 °C. Once dissolved, dilutions were made in 1 M HNO3 to obtain working concentrations of ~ 200 μg mL−1. For the experiments presented here, samples were further prepared/diluted in 2% HNO3 (Fisher Optima, Pittsburgh, Pa, USA). A natural U sample, CRM 129-A, was provided by High Purity Standards (Charleston, SC, USA) as a stock 1 μg mL−1 and was treated as an unknown during these experiments. Table 2 shows the 235U/238U values for each of the samples used in these experiments. For the sake of this benchmarking study, only the 235U/238U ratio was compared. Beyond the solid CRM dissolution procedure described above, each instrument operator was provided with a 5-mL ampule containing the unknown solution (CRM 129-A). Dilutions were made for each specific instrument, as necessary, using a 2% HNO3 solution, with final concentrations for each of the instrumental methods shown in Table 3.
LS-APGD-Orbitrap Mass Spectrometry
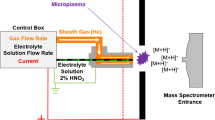
The LS-APGD [15, 31,32,33,34] microplasma was interfaced to a Thermo Scientific Q Exactive Focus (San Jose, CA, USA) mass spectrometer (Figure 1). The ion source consists of a solution grounded cathode and a solid stainless steel counter electrode (SS, weldable feedthrough, MDC Vacuum Products, LLC, Hayward, CA, USA) to which a potential is applied from a Spellman high-voltage power supply (SL-60, Spellman High Voltage Electronics Corporation, Hauppauge, NY, USA). The solution electrode has an outer capillary (316 SS, 0.8 mm i.d., 1.6 mm o.d., IDEX Health and Science, Oak Harbor, WA, USA), which provides a He cooling/sheath gas and a nested inner capillary (280 μm i.d., 580 μm o.d., Restek Corporation, Bellefonte, PA, USA) that delivers the sample solution to the plasma. The gas flow rate was controlled using a mass flow controller (Alicat Scientific, Inc. Dallas, TX, USA). Solution was delivered using two Fusion 100 syringe pumps (Chemyx, Stafford, TX, USA), set in parallel with a six-port Rheodyne 7125 injection valve (Valco Instruments Co. Inc., Houston, TX, USA) used to switch between pumps. The first syringe pump was used to deliver the sample while the second syringe was used to deliver a sodium citrate solution which was used to sustain the plasma and to wash U from the system to reduce carryover. Nominal total U concentrations were 100 ng mL−1. Similar to previous isotope ratio studies, the operating parameters for the LS-APGD were current = 30 mA, gas flow rate = 0.5 L min−1, and solution flow rate = 30 μL min−1 [18].
There was no modification, including changes to ion optic potentials or capillary temperatures, to the Q Exactive Focus other than removing the standard electrospray/atmospheric pressure chemical ionization sources (ESI/APCI) [18, 33]. The Q Exactive Focus was controlled by the Thermo Tune operating system and data acquisition software and operated in the positive ion mode. Acquisitions consisted of 100 scans with each scan made up of 10 microscans. Each microscan is comprised of the injection of an ion packet into the Orbitrap where a single transient is acquired. Once the 10 transients (corresponding to the 10 microscans) are acquired, the transients are averaged and Fourier transformed to produce a single spectrum. Ion packets were formed by injecting ions into the C-Trap for a fixed period of 100 ms in all cases. U signals were measured by using the peak area measurement provided by the Thermo software Xcalibur™ Qual Browser. As in previous work, all U measurements were carried out by monitoring the dioxide cation (UO2+), as this was the predominant U-containing ion present in the spectra [15,16,17,18]. (Interestingly, the atomic U+ is most prominent when sampling the microplasma with a conventional Paul-type trapping instrument [20].) The ability of the Orbitrap to provide much higher mass resolution than the other instruments is incredibly useful. In this application, the Orbitrap resolution was set to m/Δm = 70,000, which was the maximum for the Q Exactive Focus. Figure 2 shows a representative mass spectrum, taken while analyzing CRM U500, showing all four U isotopes present in the sample. As can be seen, the Orbitrap has the dynamic range necessary to measure the 234U and 236U isotopes with a high degree of accuracy and precision. Additionally, the virtues of the instrument’s resolving power are demonstrated as the 236U16O2+ peak is baseline-resolved from the 235U16O17O+ peak allowing for accurate isotope ratio measurements to be made. Without this level of mass resolution (as in the case of the other mass analyzers employed here), the 235U16O17O+ signal would be an isobaric interferent of the 236U16O2+, resulting in a 3 – 4× overestimation of the 236U fraction, which is generally corrected.
A number of mechanisms exist for reduction/removal of concomitant ions from the analyzed ion beam and also improving the isotope ratio precision and accuracy for the LS-APGD/Orbitrap pair [18]. In order to reduce concomitant molecular ions that interfere with signals of interest, in-source collision-induced dissociation (CID) and higher-energy collision-induced dissociation (HCD) were implemented. As common in many atmospheric pressure ionization instruments (e.g., ESI-MS), the in-source CID takes place between the end of the ion transfer capillary and the exit lens of the ion source interface while HCD takes place in a multi-pole collision cell (with N2 as the target gas) as is common in tandem MS platforms. For these experiments, the CID energy was set to 70 eV while the HCD was set to 100 eV. In order to improve the measurement precision and accuracy, the quadrupole mass filter was used to eliminate concomitant ions from entering the C-trap prior to injection into the Orbitrap. For this work a quadrupole range from m/z = 243.5 to m/z = 293.5 was used. Additionally, a digitization range of 10 Da, from m/z = 263.5 to m/z = 273.5, was employed.
Inductively Coupled Plasma-Sector Field Mass Spectrometry
While multi-collector ICP-MS offers superb isotope ratio analysis, scanning SFMS are also widely used to complete these measurements when the ultimate precision is not required. The Thermo Fisher Scientific (Bremen, Germany) Element XR ICP-SFMS was employed in these efforts. This SF-ICP-MS is configured such that the auto-sampler, plasma torch box, and sample/skimmer cones are housed within a radiologically controlled open front hood such that any radiological contamination is contained. The ion focusing mechanism and detector is located outside the radiological enclosure. Here, an Elemental Scientific Inc. (Omaha, NE, USA) PrepFast auto-sampler was employed for sampling into the ICP-SFMS. A MicroFlow PFA-ST nebulizer (Elemental Scientific) was employed for nebulization with an uptake solution flow rate of 200 μL min−1 and argon flow rate of 1.0 mL min−1. For improved stability, the Elemental Scientific stable sample introduction (SSI) dual quartz spray chamber was employed as it is ideal for isotope ratio measurements. The SSI spray chamber was housed within an Elemental Scientific PC3 Peltier-cooled (2 °C) inlet system. The Peltier cooler reduces the water/solvent vapor loading on the plasma thus improving stability and isotope ratio measurement performance [13, 14]. Prior to analysis, the instrument was tuned with a 1-pg mL−1 multi-element solution with an emphasis placed on measurement precision of the 238U isotope. Based on previous isotope ratio experience on this instrument, samples were diluted such that only the ion counting mode of the secondary electron multiplier was employed, which has generic limits of detection in the sub-fentogram per gram arena. Nominal total U concentrations were 1 ng mL−1. Routine peak jumping on a single collector was employed at low resolution and flat-topped peaks. For these experiments, 50 ms was used for each of the samples analyzed. The scan rate was based set to allow for 100 scans to be taken over a 2-min period. For the experiments presented here, the resolution (R = m/Δm) was ~ 300.
Inductively Coupled Plasma-Quadrupole Mass Spectrometry
Quadrupole ICP-MS is most often employed for isotope ratio measurements not requiring high precision/accuracy, utilizing the system simplicity and analysis speed of that platform. In addition, this offers a lower-cost method for prescreening samples and running samples in heavy matrixes prior to performing multi-collector ICP-MS/TIMS measurements. A Thermo Fisher Scientific X-Series 2 ICP-QMS (Breman, Germany) was used for this study. This quadrupole-based ICP-MS was employed such that the system entirety, due to its small footprint, was contained in a radiologically controlled open front hood to minimize personnel contamination. Similar to the sector field, an ESI auto-sampler, with a Mircoflow PFA-100 nebulizer was employed. A traditional cyclonic spray chamber was utilized, housed within an Elemental Scientific Inc. PC3 Peltier-cooled (2 °C) inlet system. To mimic sampling conditions, an uptake solution flow rate of 200 μL min−1 and argon flow rate of 1.0 mL min−1 were used. Here, samples were diluted such that the total U concentration was 10 ng mL−1, as the nominal detection limit of the X-Series 2 is sub-nanogram per gram. The instrument operates with unit mass resolution. Prior to operation, the instrument was tuned using a 1-μg mL−1 multi-element tune solution including depleted U optimized to a mass of 238 amu. Masses were monitored using a real-time display (RTD) and peak jumping to insure maximum sensitivity while obtaining a uniform mass response across the isotopic range. Samples were diluted into a range such that only the ion counting mode of the secondary electron multiplier was used for measurement. For these experiments, 10 ms was used for each of the samples analyzed. The scan rate was based set to allow for 100 scans to be taken over a 2-min period.
Thermal Ionization Mass Spectrometry
For low uncertainty isotope ratio measurements, TIMS instruments are the benchmark method in nuclear forensics. A Thermo Fisher Scientific Triton (Breman, Germany) multi-collector was employed for high precision isotope ratio measurements of the U isotopes. The Triton instrument has a resolution m/Δm ~ 450. The commonly used total evaporation (TE) method was employed with a double-filament (rhenium) configuration, whereas the U samples analyzed were deposited on one of the two filaments [28]. The sample-loaded filament is placed in proximity (~ 1 mm) to the ionizing filament. Samples were diluted in 1 M HNO3 to a concentration of 200 μg mL−1. For each sample, approximately 200 ng was deposited onto the center of a Re filament and was dried to a residue by ramping up the filament amperage following ASTM Test Method C1672-17 [35]. The filaments were then loaded onto the sample turret, placed into the sampling chamber, and pumped down to < 5 × 10−7 mbar. Prior to the start of the analysis, liquid nitrogen was added to the cold trap to maintain the desired vacuum level during ionization. The uranium isotope ratio protocol has been previously described [36]. The sample data was collected with the 235U and 238U intensities summed to a final detector voltage of 6 V, with a 1-s integration time until the response fell below a predefined target intensity. The amplifier associated with the TIMS instrument used during this experiment employed a dropping resister of 1011 Ohms. During analysis, all of the isotopes 234U, 235U, 236U, and 238U were measured simultaneously by employing static Faraday cups (fixed on the individual isotopes) which continually measure the ion signal throughout the analysis. The TE method produces good abundance measurements for the major isotopes 235U and 238U. Once the data was collected, isotope ratio values are determined based in the integrated signal intensities.
Benchmarking Analysis/Acquisition
For each of the plasma-based instruments (LS-APGD-Orbitrap, ICP-SFMS, and ICP-QMS), individual samples were analyzed with triplicate scans (3× of n-scans) and an average value was reported. The samples were analyzed in triplicate with alternating blanks in between such that the sequence, for instance, would consist of blank, CRM U-100, blank, CRM U-100, blank, CRM U-100. In order to correct for mass bias, a correction factor (Cf) was calculated using CRM U-500 system in the comparison across different uranium enrichment levels and in the case where CRM U-010 was treated as an unknown. The correction factor was determined by first calculating the correction factor per mass unit using Eq. 1:
where \( {\overline{r}}_s \) is the average of the measured ratios, \( \widehat{b} \) is the externally provided estimate of the true ratio of the reference material, and 3 is the number of mass units between the measured isotopes (235U and 238U). In order to calculate the corrected ratio \( {\overline{r}}_{\mathrm{cu}} \), Eq. 2 was used:
Correction factors per mass unit for each instrument can be seen in Table 4.
Due to the nature of TIMS instruments, there was no washout period or blank between samples. In this case, each sample was analyzed four times and an average value was reported. The turret had a comparator standard run at the beginning, twice in the middle and end of the turret. The reason for running the comparator standard throughout the experiment is to constrain the uncertainty of the correction factor that can arise to due to mass fractionation, which has a large associated uncertainty [24]. The comparator standard, CRM U-500, was used to estimate the mass fractionation correction factor and was applied using Eq. 1. The other samples that were run on the turret were corrected using the comparator standard and % relative deviation’s (RD) of the major ratio were plotted on quality control charts to ensure they were within pre-set limits. The comparator standard is self-correcting and should have a %RD of 0.000. Samples and standards (not including the comparator) were randomly analyzed throughout the turret.
In order to compare the measurement uncertainty between instruments and to the ITVs, the systematic uncertainty u(s), which represents the measurements accuracy, and the random uncertainty u(r), which represents the measurement precision, are presented for each analysis. The systematic uncertainty, u(s), and random uncertainty, u(r), are combined in to give a combined (total) standard uncertainty (uc) (Eq. 3).
where u(s) is given by the % relative deviation (% RD) and u(r) is given by the % relative standard deviation (% RSD) of the measurement [4].
Comparing System Operations
In order to compare the practical aspects of analysis and performance of the LS-APGD-Orbitrap system to the traditional elemental techniques presented here, four of the CRM samples were analyzed under the system operation metrics compiled in Table 3. The primary differences between the systems are the material consumed per sample and the total time required for measuring all of the samples. Both ICP instruments operate in a similar manner, with the ICP-SFMS requiring less overall sample due to greater sensitivity. While the sample flow rate of the LS-APGD-Orbitrap is only 30 μL min−1, compared to 200 μL min−1 for the ICP instruments, the time required to analyze each CRM is three times as long. One avenue to decrease the analysis time of the LS-APGD-Orbitrap is to decrease the resolution of the measurement from 70,000 to a lower resolution setting. By doing this, the time each ion packet is analyzed in the Orbitrap is decreased. A preliminary study showed that by reducing the resolution on the Q Exactive Focus from 70,000 to 20,000, the time required to take a single acquisition was reduced from 4.5 to 2.35 min. It is not clear what effect using a lower resolution will have on the measurement precision or accuracy; however, previous research presented in Hoegg et al. [17], using an Exactive instrument (different platform) and a resolution of 20,000, achieved similar results for natural and low enriched U samples. This increase in per sample analysis time increases the material consumed per sample. Two advantages that the LS-APGD has over ICP instruments is the use of relatively low gas flow rates and the fact that the LS-APGD operates in a total consumption mode eliminating waste streams. Compared to ICP sources, which use 1 L min−1 of Ar to nebulize the sample and 16 L min−1 to maintain the plasma, the LS-APGD operates using a total gas flow rate of 0.5 L min−1, He. When the total measurement times are compared, the time the LS-APGD-Orbitrap takes to complete the analysis is far longer than the traditional techniques; however, the majority of this time is taken up by the washout period, which is required to eliminate U carryover from within the ion transfer capillary of the Exactive instrument. The long pre-analysis time required by the TIMS includes the evacuation of the sample chamber to reach the vacuum levels discussed above. By improving the washout protocol of the LS-APGD-Orbitrap, it is expected that the total time required for measuring the four CRMs should fall below the time required for TIMS analysis.
Results and Discussion
Enriched Uranium Analysis Using the LS-APGD-Orbitrap System
As previously described, the ability of the LS-APGD-Orbitrap system to report the values of minor isotopes is hindered by a noise (background) deletion step completed automatically during the data processing [15,16,17,18]. Experience suggests that the system has a pre-set threshold (appearing to be a percentage of the base peak in the selected digitization window) and deleting all information below this threshold; subsequently, reporting a “0” background at all masses below that value. This leads to the generation of a bias that favors the more abundant isotope as smaller fractions of those signals are subtracted. More importantly, in the case of measuring disparate abundance isotopes, this leads to reports of no signal for low abundance isotopes. When analyzing natural abundance U samples, this leads to the deletion of peaks generated from 234UO2+ and 236UO2+ ions, representing isotopic fractions of 5.2962 × 10−3 % and 9.7 × 10−6 % of the CRM 129A sample, respectively. This deletion step is illustrated in Figure 3, where the threshold is drawn at ~ 0.007 % of the major ion signal, 238UO2+ (based on previous experience). As can be seen, the signature ion for the 234UO2+ is below the threshold, while an appreciable fraction of the 235U signal would be subtracted. (Note, the 236 UO2+ signal would be infinitesimally small on this scale.) Because the threshold value is a percentage of the base peak intensity, there is no improvement when increasing the total concentration of the U sample. Additionally, the presence of concomitant ions and the resulting spectral noise exasperates the problem by increasing the threshold. To be clear, the exact procedure for setting the threshold is unknown and there is no process in the standard Thermo Tune Software to turn this data processing step off. Ultimately, this noise deletion step, which is unique to the Exactive platform, limits the dynamic range of the Orbitrap.
Previously, the deletion of minor isotope signals has limited the LS-APGD-Orbitrap system to only reporting the 235U/238U ratio for the samples run, including depleted, natural, and low enriched U. In the case of highly enriched U samples, however, the isotopic abundance of 234U and 236U isotope increases in relation to the major isotope (238U) allowing for reporting the 234U/238U and 236U/238U ratios. Based on the isotopic compositions, 234UO2+ was observed in CRMs, U-100, U-500, and U-800, while the 236UO2+ signal was only observed for the two most highly enriched CRMS, U-500 and U-800. As an example of the improvement in isotope ratio performance related to the background deletion threshold, the 234U/238U precision of the measurements improves appreciably from 4.8 to 0.1 % RSD in simply moving from U-100 to U-500, with the accuracy improving from an error of ~ 50 % relative to ~ 10 %. Due to the fact that the 234U and 236U isotopes were not consistently seen across all of the samples, the remaining discussion will focus on using the 235U/238U ratio for comparison purposes. Indeed, this is the primary ratio of interest in most of the relevant applications.
Isotope Ratio Performance Comparisons Among Methods with Increasing Levels of 235U Enrichment
In order to establish a preliminary benchmark of the ability of the LS-APGD-Orbitrap system to measure isotope ratio characteristics over a wide range ratio values, the system performance was compared against elemental mass spectrometers (TIMS, ICP-SFMS, and ICP-QMS) using CRMs with increasing 235U isotopic composition. The CRMs are listed in Table 2 and ranged from 1 to 80 % 235U. Table 5 shows the results of the study. For each of the plasma instruments, the CRMs were run during a single session from low enriched (CRM U-010) to highly enriched (CRM U-800), with CRM U-500 used to determine a mass bias correction factor for each instrument. The TIMS analysis was completed as discussed in the “Materials and Methods” section. The plasma sources (the ICP and LS-APGD) were run so that three acquisitions made up a single sample (data point) with washouts in between each data point. Due to U carryover in the ion transfer capillary of the Exactive mass spectrometer, a washout protocol was developed for the LS-APGD-Orbitrap system. This protocol involved passing a 10-μg mL−1 sodium citrate solution as the electrolyte solution. This was adapted from Francis et al. [37], who used citrate solutions to remove U contamination from steel samples that had undergone corrosion in the presence of U in equipment and buildings. Their results showed that by using a citrate solution, up to 63 % of U was removed from the surface of the steel samples. While effective at reducing carryover by an appreciable extent, a more thorough, independent study is required to assess the quantitative performance and to ascertain the mechanism by which the citrate alleviates U carryover from the LS-APGD-Orbitrap system.
When the uncertainty results are compared in Table 5, the LS-APGD-Orbitrap and the TIMS instruments are the only two that meet the ITVs for each of the sample types. The ICP-SFMS instrument was able to meet the ITVs at lower enrichment values, but was slightly above the ITVs for the highly enriched CRM U-800 sample analysis. Based on these results, the ICP-QMS instrument was unable to meet the ITVs for any of the samples analyzed here. As stated in the “Materials and Methods” section, a dwell time of 10 ms per isotope was used, which is typical of this laboratory. It might be expected that the measurement precision would increase with longer dwell times and larger sample sets. When the uncertainty values are compared across the TIMS, ICP-SF-MS and LS-APGD-Orbitrap instruments, the TIMS was consistently below 0.02 % RSD, with a majority of the combined uncertainty, (uc), coming from the systematic uncertainty component, u(s), which is a measurement of the system accuracy. The uncertainty for the ICP-SFMS was consistent across the analysis for both the systematic uncertainty component, u(s), and random uncertainty component, u(r). Interestingly, while the systematic uncertainty component, u(s), contributed the most to the combined uncertainty, (uc), for both the TIMS and the LS-APGD-Orbitrap system, in the case of the ICP-SFMS, the reverse is true. This trend may be explained by the fact that the TIMS and LS-APGD-Orbitrap simultaneously sample/measure the isotopes, while the ICP-SFMS instrument sequentially scans each isotope. More research is needed to substantial this hypothesis. The random uncertainty component, u(r), of the ICP-SFMS system was approximately 0.08 % for each of the samples except for the CRM U-500, the sample used to determine the correction factor. When the uncertainty of the LS-APGD-Orbitrap system is examined, a decrease in the systematic uncertainty component, u(s), is observed in the analysis of the CRM U-800 relative to the lesser enriched samples. A decrease in the overall uncertainty would be expected as the 235U isotope abundance increases, based on statistical arguments. This is reflected in the ITV requirements becoming stricter (lower uncertainties) at higher enrichment values. It is also noted that in the case of CRM U-800, the major isotope is 235U. This means that the noise deletion step for the LS-APGD/Orbitrap would create a bias in favor of the 235U isotope which may be driving the value closer to the expected value. The random uncertainty component, u(r), for the LS-APGD-Orbitrap system was below 0.032 % RSD for the sample types run during this analysis. Based on these preliminary results of analyzing highly enriched U samples, and given the range of samples tested, the LS-APGD shows significant promise as a tool in the nuclear safeguards arena.
Isotope Ratio Performance Comparisons Among Methods for an Unknown Sample
The ability of a system to correctly measure an unknown is vital during process of instrument development and method validation. In order to benchmark the LS-APGD-Orbitrap system against the traditional elemental mass spectrometers for this purpose, CRM 129a was treated as an unknown. While a similar experiment was previously completed by Marcus et al. [17], the unknowns and known were all natural U samples having similar 235U/238U values. In this experiment, the low enriched U sample, CRM U-010, was used to determine a correction factor providing a more realistic test. Table 6 shows the results of this study.
When the combined uncertainty values, (uc), are compared to the ITVs in Table 1, all of the systems, except for the ICP-QMS instrument, fall below the ITVs for natural U. Furthermore, the random uncertainty component, u(r), for the CRM U-010 measurement also fell below the ITV. However, the systematic uncertainty component, u(s), was not calculated because the CRM U-010 was used to provide a correction factor for the unknown (CRM 129A). When the individual uncertainty components of the LS-APGD-Orbitrap measurements are compared to the target values, the systematic uncertainty component, u(s), is slightly higher than the target value by − 0.0009 %. The fact that the systematic uncertainty component, u(s), is 4× larger than the random uncertainty component, u(r), and that the measured value falls below the real value for CRM 129A further suggests that the noise deletion step is creating a bias towards the more abundant 238U isotope. This may also suggest that a CRM composition closer to the unknown may be necessary when measuring samples where the 235U is below a certain level in order to better correct for the noise deletion step. A more thorough investigation, including the use of stable isotope standards, to determine the full effect of the noise deletion step is certainly warranted. Comparing the random uncertainty component, u(r), generated by the different systems, the LS-APGD-Orbitrap compares remarkably well especially when compared to the ICP-QMS. As expected, the TIMS provided results with the lowest uncertainty, closely followed by the ICP-SFMS. Based on these results, the LS-APGD compares favorably with the TIMS and scanning SF instruments; however, depleted and natural U samples suffer from high systematic uncertainties (u(s)) for this method, possibly due to the background subtraction step that has no analogy in any of the other instrument methods tested here.
Comparisons of Practical Aspects of Operation Among Methods
The LS-APGD-Orbitrap System offers many practical advantages over traditional elemental ion sources. Table 3 presented many of the operational differences between the systems; however, other considerations including system resolution are not highlighted. As demonstrated in Figure 2, the Orbitrap offers high-resolution spectra that can eliminate isobaric interferences. This is a significant advantage when looking at oxides, as shown above, or resolving isotopes of varying elements. Case in point for potential nuclear forensics applications, the Orbitrap would be able to easily resolve signals from 238U and 238Pu. This would not be possible on the other platforms. The fact that the Orbitrap footprint makes it suitable to be placed in a radiological hood offers a large amount of flexibility when choosing an analysis method. For comparison, the ICP-QMS employed in this study has a footprint of 43″ × 25″ × 30″. The ICP-SFMS and TIMS instruments used are very much larger, 68″ × 880″ × 55″ and 41″ × 90″ × 64″ respectively. Generally speaking, the LS-APGD-Orbitrap platform (footprint of 36″ × 33″ × 37″) is directly comparable to the ICP-QMS. In terms of weight, which becomes vital to the transportation of instrumentation to field deployable arenas, the LS-APGD-Orbitrap (~ 380 lb) is again more in line with the ICP-QMS (~ 330 lb) and significantly lighter than the ICP-SFMS (~ 1500 lb), not including the need for a substantial Ar supply, and the TIMS (~ 3700 lb).
Previous research has already highlighted the flexibility offered by using the LS-APGD ion source. Zhang et al. demonstrated the ability of the LS-APGD to provide both elemental and molecular analysis of uranyl species [20]. Elemental speciation, such as knowing the nature of ligands on the U, can provide a plethora of information regarding chemical reactivity and transport of U in the environment. While the atomic MS systems described here (e.g., TIMS, ICP-QMS, and ICP-SFMS) are unequivocally employed for elemental/isotopic analysis, the ability to perform molecular MS is not feasible. An advantage to the Orbitrap platform is that it is routinely used for molecular based MS and the LS-APGD has previously illustrated the ability to analyze a range of organic compounds [19]. One could imagine having a single unit which could provide molecular based measurements as well as provide high-fidelity isotopic determinations, as demonstrated here.
Conclusions
This study was aimed at providing a preliminary comparison between the LS-APGD-Orbitrap system and traditional elemental mass spectrometry systems with respect to their performance in isotope ratio determinations of uranium. Not only did these experiments show the first results of analyzing highly enriched U samples with the LS-APGD-Orbitrap system, they also demonstrate the observation of the 234U and 236U isotopes for the first time. Delineation of the error sources across the different MS methods was very enlightening in terms of fundamentals of the measurement processes and analytical utility. The use of CRMs of increasing uranium enrichment points to biases induced by the automatic background correction process as the systematic uncertainty component (u(s)) improves with increased 235U content, while the random uncertainty component (u(r)) remains fairly consistent. The combined uncertainty of the relatively new LS-APGD-/Orbitrap coupling was very competitive with the benchmark ICP-SFMS and TIMS approaches, suggesting appreciable opportunities for future advances and applications.
Based on these results, a critical next step is determining the source of carryover in the system, in order to reduce washout time and ideally decrease the % RD of the measurements. Additionally, as the research into using the Orbitrap platform for isotope ratio analysis progresses, it has become more evident that the noise deletion step must be addressed going forward. While uranium has been a primary focus, expanding the range of elements analyzed to include plutonium and other related radionuclides, as well as stable isotope measurements, is certainly warranted. With respect to overall uncertainty when measuring isotope ratios, the system compared well with the ICP-SFMS and TIMS instruments, while it was shown to be measurably better than the ICP-QMS instrument. Future isotope ratio method developments using the LS-APGD/Orbitrap instrument will include analysis of variance (ANOVA) in order to provide more detailed understanding of sources of error and their relative impacts. When comparing the LS-APGD/Orbitrap system to the traditional elemental mass spectrometers, it is clear that several advantages exist. The ability to have a benchtop instrument capable of fitting in a radiological hood, while providing high mass accuracy and high resolution, provides for a level of flexibility in terms of the complexity of samples analyzed. Additionally, reducing consumables as well as eliminating waste streams allows for easy adoption in field deployable environments. Ultimately, given the level of accuracy and precision demonstrated here, it is clear that the LS-APGD-Orbitrap system warrants further investigation as a tool in nuclear safeguards.
References
Moody, K.J., Grant, P.M., Hutcheon, I.D.: Nuclear Forensic Analysis. CRC Press, Boca Raton (2014)
Stanley, F.E., Stalcup, A.M., Spitz, H.B.: A brief introduction to analytical methods in nuclear forensics. J. Radioanal. Nucl. Chem. 295, 1385–1393 (2013)
Kristo, M.J., Tumey, S.J.: The state of nuclear forensics. Nucl. Instrum. Meth. B. 294, 656–661 (2013)
Zhao, K., Penkin, M., Norman, C., Balsley, S., Maryer, K., Peerani, P., Pietri, C., Tapodi, S., Tsutaki, Y., Boella, M. Renha, G., Kuhn, E.: International Target Values 2010 for Measurement Uncertainties in Safeguarding Nuclear Materials. ESARDA Bulletin, 3–24 (2010)
Byerly, B.L., Stanley, F., Spencer, K., Colletti, L., Garduno, K., Kuhn, K., Lujan, E., Martinez, A., Porterfield, D., Rim, J., Schappert, M., Thomas, M., Townsend, L., Xu, N., Tandon, L.: Forensic investigation of plutonium metal: a case study of CRM 126. J. Radioanal. Nucl. Chem. 310, 623–632 (2016)
Magara, M., Hanzawa, Y., Esaka, F., Miyamoto, Y., Yasuda, K., Watanabe, K., Usuda, S., Nishimura, H., Adachi, T.: Development of analytical techniques for ultra trace amounts of nuclear materials in environmental samples using ICP-MS for safeguards. Appl. Radiat. Isot. 53, 87–90 (2000)
Qiao, J., Lagerkvist, P., Rodushkin, I., Salminen-Paatero, S., Roos, P., Lierhagen, S., Jensen, K.A., Engstrom, E., Lahaye, Y., Skipperud, L.: On the application of ICP-MS techniques for measuring uranium and plutonium: a Nordic inter-laboratory comparison exercise. J. Radioanal. Nucl. Chem. 315, 565–580 (2018)
Manard, B.T., Derrick Quarles, C., Wylie, E.M., Xu, N.: Laser ablation-inductively couple plasma-mass spectrometry/laser induced break down spectroscopy: a tandem technique for uranium particle characterization. J. Anal. At. Spectrom. 32, 1680–1687 (2017)
Kappel, S., Boulyga, S.F., Dorta, L., Günther, D., Hattendorf, B., Koffler, D., Laaha, G., Leisch, F., Prohaska, T.: Evaluation strategies for isotope ratio measurements of single particles by LA-MC-ICPMS. Anal. Bioanal. Chem. 405, 2943–2955 (2013)
Kappel, S., Boulyga, S.F., Prohaska, T.: Direct uranium isotope ratio analysis of single micrometer-sized glass particles. J. Environ. Radioact. 113, 8–15 (2012)
Erdmann, N., Betti, M., Stetzer, O., Tamborini, G., Kratz, J.V., Trautmann, N., van Geel, J.: Production of monodisperse uranium oxide particles and their characterization by scanning electron microscopy and secondary ion mass spectrometry. Spectrochim. Acta B. 55, 1565–1575 (2000)
Jakubowski, N., Feldmann, I., Stuewer, D.: Analytical improvement of pneumatic nebulization in ICP-MP by desolvation. Spectrochim. Acta B. 47, 107–118 (1992)
Boulyga, S.F., Heumann, K.G.: Determination of extremely low U-236/U-238 isotope ratios in environmental samples by sector-field inductively coupled plasma mass spectrometry using high-efficiency sample introduction. J. Environ. Radioact. 88, 1–10 (2006)
Park, R., Lim, S.H., Han, S.H., Lee, M.Y., Park, J., Lee, C.G., Song, K.: Improvement of measurement precisions for uranium isotopes at ultra trace levels by modification of the sample introduction system in MC-ICP-MS. Mass Spectrom. Lett. 7, 50–54 (2016)
Hoegg, E.D., Barinaga, C.J., Hager, G.J., Hart, G.L., Koppenaal, D.W., Marcus, R.K.: Preliminary figures of merit for isotope ratio measurements: the liquid sampling-atmospheric pressure glow discharge microplasma ionization source coupled to an Orbitrap mass analyzer. J. Am. Soc. Mass Spectrom. 27, 1393–1403 (2016)
Hoegg, E.D., Barinaga, C.J., Hager, G.J., Hart, G.L., Koppenaal, D.W., Marcus, R.K.: Isotope ratio characteristics and sensitivity for uranium determinations using a liquid sampling-atmospheric pressure glow discharge ion source coupled to an Orbitrap mass analyzer. J. Anal. At. Spectrom. 31, 2355–2362 (2016)
Hoegg, E.D., Marcus, R.K., Koppenaal, D.W., Irvahn, J., Hager, G.J., Hart, G.L.: Determination of uranium isotope ratios using a liquid sampling atmospheric pressure glow discharge/Orbitrap mass spectrometer system. Rapid Commun. Mass Spectrom. 31, 1534–1540 (2017)
Hoegg, E.D., Marcus, R.K., Hager, G.J., Hart, G.L., Koppenaal, D.W.: Concomitant ion effects on isotope ratio measurements with liquid sampling-atmospheric pressure glow discharge ion source Orbitrap mass spectrometry. J. Anal. Atom. Spectrom. 33, 251–259 (2018)
Zhang, L.X., Marcus, R.K.: Mass spectra of diverse organic species utilizing the liquid sampling-atmospheric pressure glow discharge (LS-APGD) microplasma ionization source. J. Anal. At. Spectrom. 31, 145–151 (2016)
Zhang, L.X., Manard, B.T., Powell, B.A., Marcus, R.K.: Preliminary assessment of potential for metal–ligand speciation in aqueous solution via the liquid sampling–atmospheric pressure glow discharge (LS-APGD) ionization source: uranyl acetate. Anal. Chem. 87, 7218–7225 (2015)
Manard, B.T., Gonzalez, J.J., Sarkar, A., Dong, M.R., Chirinos, J., Mao, X.L., Russo, R.E., Marcus, R.K.: Liquid sampling-atmospheric pressure glow discharge as a secondary excitation source: assessment of plasma characteristics. Spectrochim. Acta B. 94-95, 39–47 (2014)
Marcus, R.K., Burdette, C.Q., Manard, B.T., Zhang, L.X.: Ambient desorption/ionization mass spectrometry using a liquid sampling-atmospheric glow discharge (LS-APGD) ionization source. Anal. Bioanal. Chem. 405, 8171–8184 (2013)
Scigelova, M., Makarov, A.: Orbitrap mass analyzer—overview and applications in proteomics. Proteomics. 6(Suppl 2), 16–21 (2006)
Eliuk, S., Makarov, A.: Evolution of Orbitrap mass spectrometry instrumentation. Annu. Rev. Anal. Chem. 8, 61–80 (2015)
Eiler, J., Cesar, J., Chimiak, L., Dallas, B., Grice, K., Griep-Raming, J., Juchelka, D., Kitchen, N., Lloyd, M., Makarov, A., Robins, R., Schwieters, J.: Analysis of molecular isotopic structures at high precision and accuracy by Orbitrap mass spectrometry. Int. J. Mass Spectrom. 422, 126–142 (2017)
Aggarwal, S.K.: Thermal ionisation mass spectrometry (TIMS) in nuclear science and technology—a review. Anal. Methods-UK. 8, 942–957 (2016)
Mathew, K.J., Stanley, F.E., Thomas, M.R., Spencer, K.J., Colletti, L.P., Tandon, L.: Critical need for plutonium and uranium isotopic standards with lower uncertainties. Anal. Methods-UK. 8, 7289–7305 (2016)
Mathew, K.J., O'Connor, G., Hasozbek, A., Kraiem, M.: Total evaporation method for uranium isotope-amount ratio measurements. J. Anal. At. Spectrom. 28, 866–876 (2013)
Park, J.H., Jeong, K.: Experimental evaluation of the detection methods of thermal ionization mass spectrometry for isotopic analysis of ultra-trace level uranium. Microchem. J. 137, 334–341 (2018)
Boulyga, S.F., Koepf, A., Konegger-Kappel, S., Macsik, Z., Stadelmann, G.: Uranium isotope analysis by MC-ICP-MS in sub-ng sized samples. J. Anal. At. Spectrom. 31, 2272–2284 (2016)
Marcus, R.K., Davis, W.C.: An atmospheric pressure glow discharge optical emission source for the direct sampling of liquid media. Anal. Chem. 73, 2903–2910 (2001)
Marcus, R.K., Manard, B.T., Quarles, C.D.: Liquid sampling-atmospheric pressure glow discharge (LS-APGD) microplasmas for diverse spectrochemical analysis applications. J. Anal. At. Spectrom. 32, 704–716 (2017)
Marcus, R.K., Quarles, C.D., Barinaga, C.J., Carado, A.J., Koppenaal, D.W.: Liquid sampling-atmospheric pressure glow discharge ionization source for elemental mass spectrometry. Anal. Chem. 83, 2425–2429 (2011)
Zhang, L.X., Manard, B.T., Kappel, S.K., Marcus, R.K.: Evaluation of the operating parameters of the liquid sampling-atmospheric pressure glow discharge (LS-APGD) ionization source for elemental mass spectrometry. Anal. Bioanal. Chem. 406, 7497–7509 (2014)
International, A:. ASTM C1672-17, Standard Test Method for Determination of Uranium or Plutonium Isotopic Composition or Concentration by the Total Evaporation Method Using a Thermal Ionization Mass Spectrometer. ASTM International, West Conshohocken, PA (2017). http://www.astm.org, https://doi.org/10.1520/C1672-17
Mathew, K., Mason, P., Voeks, A., Narayanan, U.: Uranium isotope abundance ratios in natural uranium metal certified reference material 112-A. Int. J. Mass Spectrom. 315, 8–14 (2012)
Francis, A.J., Halada, G., Gillow, J., Clayton, C.: Mechanisms of Radionuclide-Hyroxycarboxylic Acid Interactions for Decontamination of Metallic Surfaces (2002). Final Report, Brookhaven National Laboratory. https://doi.org/10.2172/794072
Acknowledgements
Efforts in the development of the LS-APGD microplasma ionization source have been supported by the Defense Threat Reduction Agency, Basic Research Award #HDTRA1-14-1-0010, to Clemson University. High Purity Standards (Charleston, SC, USA) is acknowledged for the donation of CRM 129a.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoegg, E.D., Manard, B.T., Wylie, E.M. et al. Initial Benchmarking of the Liquid Sampling-Atmospheric Pressure Glow Discharge-Orbitrap System Against Traditional Atomic Mass Spectrometry Techniques for Nuclear Applications. J. Am. Soc. Mass Spectrom. 30, 278–288 (2019). https://doi.org/10.1007/s13361-018-2071-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-018-2071-2