Abstract
Data evaluation is a crucial step when it comes to the determination of accurate and precise isotope ratios computed from transient signals measured by multi-collector–inductively coupled plasma mass spectrometry (MC-ICPMS) coupled to, for example, laser ablation (LA). In the present study, the applicability of different data evaluation strategies (i.e. ‘point-by-point’, ‘integration’ and ‘linear regression slope’ method) for the computation of 235U/238U isotope ratios measured in single particles by LA-MC-ICPMS was investigated. The analyzed uranium oxide particles (i.e. 9073-01-B, CRM U010 and NUSIMEP-7 test samples), having sizes down to the sub-micrometre range, are certified with respect to their 235U/238U isotopic signature, which enabled evaluation of the applied strategies with respect to precision and accuracy. The different strategies were also compared with respect to their expanded uncertainties. Even though the ‘point-by-point’ method proved to be superior, the other methods are advantageous, as they take weighted signal intensities into account. For the first time, the use of a ‘finite mixture model’ is presented for the determination of an unknown number of different U isotopic compositions of single particles present on the same planchet. The model uses an algorithm that determines the number of isotopic signatures by attributing individual data points to computed clusters. The 235U/238U isotope ratios are then determined by means of the slopes of linear regressions estimated for each cluster. The model was successfully applied for the accurate determination of different 235U/238U isotope ratios of particles deposited on the NUSIMEP-7 test samples.
Similar content being viewed by others
Introduction
Particles containing radionuclides are emitted during processes related to the nuclear fuel cycle (e.g. in enrichment facilities and in nuclear reactors). The knowledge of the uranium (U) and/or plutonium (Pu) isotopic signatures of such particles is highly valuable for international safeguards [1] and nuclear forensics [2], as it helps to verify the absence of undeclared nuclear activities. International Atomic Energy Agency (IAEA) inspectors collect these particles, which typically exhibit sizes in the low micrometer range, by means of cotton swipes during routine inspections of nuclear facilities and the nearby environment [3, 4].
Considering the analysis of the sampled particles, individual analysis of single particles is preferred over bulk analysis of the entire swipe. The swipe may contain a small number of particles with hidden isotopic signatures together with a large number of particles having known or natural isotopic composition. In such a case, bulk analysis of the entire swipe would yield a ‘mixed’ U or Pu isotopic composition, and isotopic signatures of suspicious particles would eventually not be detected [1, 3]. However, it has to be stressed that bulk analysis, including the measurement of U and Pu concentrations and isotopic compositions, is equally important for verifying the completeness and correctness of States Declarations [5].
A promising technique for U isotope ratio analyses of single particles is laser ablation–multi-collector–inductively coupled plasma mass spectrometry (LA-MC-ICPMS) [6–11]. LA of single particles with sizes in the low micrometer range typically yields transient signals lasting less than 1 s.
However, transient signals are also generated by other sample introduction techniques such as high performance liquid chromatography (HPLC) [12], gas chromatography (GC) [13–15], flow-injection [16] or gold trap [17, 18]. Compared to continuous steady-state signals measured after solution nebulization, transient signals usually lead to less precise isotope ratios [12, 19], which is mainly attributed to shorter measurement times, lower signal intensities due to lower analyte concentrations introduced and isotope ratio drifts over the transient signal [20]. Moreover, precision of individual data points, which is often referred to as ‘internal’ precision in literature [14, 17], varies over the transient signal as a result of varying signal intensities. According to counting statistics [21] in which the relative standard deviation is expressed as the square root of the reciprocal of the registered counts, higher counts are yielding a smaller relative standard deviation and more precise isotope ratio data. Günther-Leopold et al. [12], for example, observed the smallest isotope ratio (point-to-point) fluctuations at the top of the peak when performing neodymium (Nd) measurements by HPLC-MC-ICPMS. In LA-MC-ICPMS analyses, improved signal-to-noise ratios can be achieved by applying a laser cell with fast washout of the generated aerosol [22]. In addition, high spatial resolution analysis is enabled as mixing of aerosol from different spots is avoided [23].
Isotope ratio drifts over the transient signal have been reported by several authors using GC [14, 24–26], HPLC [12, 27] and LA [28, 29] as sample introduction techniques for MC-ICPMS. Krupp and Donard [14] considered four effects as potential causes for the observed drifts in lead (Pb) and mercury (Hg), respectively, isotope ratio measurements by GC-MC-ICPMS. They studied instrumental mass bias, chromatographic fractionation in the GC column, a rise in the background signal during peak elution and the influence of analyte concentration and peak shape. As only an influence with respect to the peak width was identified, the authors pointed out the possibility that the relative change in analyte intensity per time might be the most pronounced effect driving the extent of the isotope ratio drift [14]. Hence and due to the fact that isotope ratio drifts were observed applying different MC-ICPMS instruments (i.e. ‘Axiom’ (Thermo, Winsford) [14], ‘Isoprobe’ (GV Instruments, Manchester) [14] and ‘Neptune’ (Thermo Fisher Scientific, Germany) [12]), it was assumed that the data acquisition system design behind the Faraday cups might lead to problems with respect to the acquisition of short, fast changing signals [14]. The same postulation was given by Dzurko et al. [24] and Günther-Leopold et al. [27]. During the simultaneous acquisition of transient signals, Faraday amplifier outputs are lagging behind input signals after a change in signal intensities. Thus, any difference in the amplifier response times leads to signal intensities that are enhanced or reduced relative to each other [29]. Hirata et al. [28] investigated the effect of fast increasing or decreasing copper (Cu) isotope ratios over a transient signal, whereat changing Cu isotope ratios were also attributed to the slow response of Faraday preamplifiers. The introduction of a correction factor enabled to minimize the systematic increase of Cu isotope ratios with prolonged LA from 3–5 ‰ to <1 ‰ [28].
A drift of Pb isotope ratios during the course of LA-MC-ICPMS measurements of fluid inclusions using a ‘Nu Plasma 1700’ MC-ICPMS (Nu Instruments Limited, Wrexham, UK) was observed by Pettke et al. [29], who again attributed this observation to Faraday amplifier response differences. The authors investigated two different signal decay functions (i.e. Tau-correction) as well as two different integration methods for the determination of accurate Pb isotope ratios of fluid inclusions, whereupon integration of single intensities over the entire transient signal was regarded as method of choice. In addition, applying Tau-correction allowed accounting for differences in Faraday amplifier responses [29]. Cottle et al. [22] observed differing detector response times with respect to Faraday detectors and ion counting multipliers (i.e. discrete-dynode secondary electron multipliers) when performing Pb/U isotope ratio measurements by means of a ‘Nu Plasma’ MC-ICPMS (Nu Instruments Limited, Wrexham, UK). Both signals rose at a similar rate, but the Faraday signal was delayed by about 0.2 s relative to the ion counting multiplier. The influence of this time-offset as well as of the Faraday amplifier response effects on the determined isotope ratios was circumvented by integrating the single measured signal intensities over the whole transient signal prior to the calculation of the isotope ratios [22].
Recently, Fietzke et al. [30] proposed a new data evaluation strategy for transient LA-MC-ICPMS signals. In their approach, strontium (Sr) isotope ratios were derived from the slope of a linear regression, with the isotope representing the numerator on the y-axis and the denominator on the x-axis. The authors highlighted several advantages such as (1) the avoidance of a subjective influence that might occur by setting integration limits, (2) the use of all data (i.e. including background data), (3) the contribution of each data point, dependent on its signal intensity, to the linear regression and (4) the detection of interferences or fractionation due to deviations from the ideal linear fit [30]. In comparison to conventional data reduction (i.e. separate background correction and calculation of 87Sr/86Sr isotope ratios for each individual point), four to five times better precision and accuracy could be achieved for LA-MC-ICPMS 87Sr/86Sr isotope ratio measurements of a carbonate sample. Moreover, the authors stated that the 87Sr/86Sr isotope ratios determined in their study were almost as precise as those measured by means of conventional liquid nebulization MC-ICPMS [30]. Epov et al. [13] compared three data reduction methods (i.e. ‘peak area integration’, ‘point-by-point’ and ‘linear regression slope’ method) for the determination of Hg isotopic compositions by means of GC-MC-ICPMS. It was demonstrated that the method using the slope of a linear regression typically yielded more precise and accurate δ Hg values than the other strategies. In addition, Rodríguez-Castríllon [31] applied this new data evaluation strategy for the determination of Sr and Nd isotope ratios by means of MC-ICPMS coupled to on-line liquid chromatography.
The above-discussed publications dealing with isotope ratio determinations from transient signals illustrate well that data treatment is a crucial step. However, the analytical community strives towards new data evaluation strategies in order to reduce the relative difference to the certified value and the uncertainty of isotope ratios from transient signals as was recently shown by various authors [13, 30, 31]. This work aimed at investigating the applicability of different data reduction strategies for the computation of major U isotope ratios (i.e. 235U/238U) from single particle measurements and to provide accurate measurement results on single particles with combined uncertainty and traceability. The usefulness of an innovative evaluation approach, termed ‘finite mixture model’ [32], was demonstrated by applying this approach for the accurate determination of multiple U isotopic signatures measured in single particles.
Experimental
Reagents and certified reference materials
Certified reference materials (CRM) that are certified with respect to their U isotope ratios—IRMM-184 (European Commission-JRC, Institute for Reference Materials and Measurements, Geel, Belgium [33]), CRM U030-A (New Brunswick Laboratory, US Department of Energy, Washington, DC, USA [34]) and CRM U500 (New Brunswick Laboratory, US Department of Energy, Washington, DC, USA [35])—were used for the determination of external correction factors for correcting mass bias. The certified reference materials were introduced by solution nebulization after dilution to concentrations less than 10 ng g−1 by 1 % (m/m) HNO3. One percent (m/m) HNO3 was prepared by diluting 65 % (m/m) HNO3 (Merck KGaA, Darmstadt, Germany) with ultrapure water (18 MΩ cm at 25 °C; PURELAB® Classic, Veolia Water Systems Austria GmbH, Wien, Austria at BOKU Vienna; Milli-Q® Element, Millipore, Millipore Corporation, Billerica, MA, USA at ETH Zurich). Ultrapure water and 65 % (m/m) HNO3 were purified by sub-boiling distillation (Savillex Corporation, Eden Prairie, MN, USA at BOKU Vienna; DuoPUR, Milestone S.r.l., Italy at ETH Zurich) prior to use.
The following single uranium oxide particles, which are certified for their U isotopic compositions, were measured: 9073-01-B (UO2 · 2 H2O particles, European Commission-JRC, Institute for Reference Materials and Measurements, Geel, Belgium [36]), CRM U010 (U3O8 particles, New Brunswick Laboratory, US Department of Energy, Washington, DC, USA [37]) and NUSIMEP-7 test samples (U3O8 particles, Nuclear Signatures Interlaboratory Measurement Evaluation Programme, European Commission-JRC, Institute for Reference Materials and Measurements, Geel, Belgium [38]). NUSIMEP-7 was an interlaboratory comparison (ILC) organized by the Institute for Reference Materials and Measurements. Participating laboratories received two test samples with U particles with undisclosed U isotope ratios. The ‘single’ deposition sample had one U isotopic composition, whereas the ‘double’ deposition sample had two different isotopic compositions. The average diameter of the NUSIMEP-7 samples was reported to be 0.327 ± 0.139 μm [38], whereas the particle sizes of 9073-01-B and CRM U010, which were determined by means of scanning electron microscopy at the TU Vienna (Quanta 200, FEI, Eindhoven, The Netherlands), ranged from about 1 to 5 μm. The used particle reference materials are considered to be representative for particles collected by IAEA inspectors using swipe sampling, even though they are exhibiting a broad particle size distribution. However, swipe samples may contain particles of different origins, and thus of different chemical compositions and sizes. The certified isotope ratios of the used CRMs are listed in Table 1.
Particle preparation for LA-MC-ICPMS analyses
Particle preparation for LA-MC-ICPMS analyses of 9073-01-B (UO2 · 2 H2O) and CRM U010 (U3O8) particles was performed in a class 100 clean room at the IAEA Safeguards Analytical Laboratory in Seibersdorf, Austria. The 9073-01-B and CRM U010 particles were distributed on cotton swipes, from which they were sampled onto silicon planchets by means of a vacuum impactor.
Silicon planchets are typically used for particle preparation for subsequent LA-MC-ICPMS analysis in our laboratory, as particles are more easily identifiable than with carbon planchets with the observation of the LA system. However, the particles of the NUSIMEP-7 samples were already distributed on graphite planchets, which are routinely used for particle preparation for secondary ion mass spectrometry (SIMS). The potential formation of carbon-containing cluster ions was monitored by measuring blank planchets, but no interferences were detected. The NUSIMEP planchets used in this work were found to have been contaminated with enriched U during sample handling in a laminar flow bench, typically used for U sample preparation. Nonetheless, these planchets were analyzed to demonstrate the potential of the developed evaluation method to identify multiple isotopic compositions in mixed samples.
All planchets were covered with a colourless, commercially available nail polish, which was mixed 1:1 (v/v) with acetone (acetone p.a., Merck KGaA, Darmstadt, Germany). This step ensured that the particles were not moved during the LA process.
LA-MC-ICPMS
U isotope ratio measurements of 9073-01-B and CRM U010 single U particles were accomplished at the ETH Zurich with a double-focusing high-resolution sector field MC-ICPMS (‘Nu Plasma HR’, Nu Instruments Limited, Wrexham, UK). The instrument was coupled with a femtosecond (fs) LA system operating at a wavelength of 795 nm. The fs LA system uses a chirped pulse amplification Ti-sapphire-based laser system (Legend, Coherent Inc., Santa Clara, CA, USA). A large ablation cell with fast washout (i.e. decrease to signal intensities as low as 0.1–1 % within 1.3–2.6 s) was used [23].
U isotope ratio measurements of the NUSIMEP-7 test samples were performed at the BOKU Vienna by coupling a nanosecond excimer-based LA system (NWR 193, ESI–NWR Division, Electro Scientific Industries, Inc., Portland, CA, USA) with a ‘Nu Plasma HR’ MC-ICPMS (Nu Instruments Limited, Wrexham, UK). The used laser cell also enabled a fast washout (i.e. decrease to signal intensities as low as 1 % within less than 1.5 s) of the generated laser aerosol.
The dark background of the planchets and the particle sizes (ca. 0.3–5 μm) hampered the identification of individual particles with the observation systems attached to the LA systems. Therefore, line or raster scans were applied.
In both cases, a desolvating nebulizer system (DSN-100, Nu Instruments Limited, Wrexham, UK) was connected in parallel to the LA system by means of a laminar flow adapter. The DSN-100 was used for solution nebulization to produce a dry aerosol for determining external correction factors. Blank correction of the solution-nebulized CRMs was accomplished by 1 % (m/m) HNO3. No solution was aspirated during LA to minimize possible blank influences during the ablation process.
Faraday cups (i.e. L1 and L3) were used for the detection of the major U isotopes 235U and 238U on both instruments.
Operating parameters are given in Table 2.
Data treatment
IRMM-184, U030-A and U500, respectively, were measured before and after the particle analyses for the determination of the external correction factor \( \mathrm{C}{{\mathrm{F}}_{{{R_{{23{5_{\mathrm{U}/}}23{8_{\mathrm{U}}}}}}}}} \), which corrects primarily for mass bias in the case of 235U/238U measurements [6].
Signal intensities of the U isotopes during LA-ICPMS measurements were recorded in time-resolved analysis mode. The blank signals were calculated from the U background signals of blank planchets (i.e. planchets with no particles but covered with nail polish). Typically, the average of up to 500 readings was used for assessing the blank signals. After blank correction, a threshold was set. Only signals higher than 3× the standard deviation of the blank of 235U (lower abundant isotope) were considered for further data processing.
Different data evaluation approaches were investigated for the calculation of 235U/238U isotope ratios of individual particles (taking into account all data of one peak per particle above a certain threshold).
‘Point-by-point’ method (PBP)
Calculation of U isotope ratios was accomplished by averaging the U isotope ratios derived from dividing individual, simultaneously acquired data points. All data points of one peak are contributing equally, independent on the signal intensity.
‘Integration’ method
235U/238U isotope ratios were calculated by dividing the signal intensities which were integrated over the selected peak area. Small count rates contribute to a lesser extent to the isotope ratio than higher count rates. This approach is commonly applied in chromatography [12, 15, 20, 26].
‘Linear regression slope’ method (LRS)
The U isotope ratios were calculated by means of the slope of a linear regression using the ‘least squares’ method of the regression analysis. The intercept was not considered as blank corrected data were used. All selected data points of one peak (i.e. >3× standard deviation of the blank) are contributing to the linear fit (i.e. y = ax), whereupon higher signal intensities are having a larger impact. Moreover, the LRS method was applied to non-blank corrected data. In this case, the intercept (i.e. y = ax + b) was considered as both the blank and the particle signal intensities (non-blank corrected) were taken into account.
‘Finite mixture model’
The ‘finite mixture model’ was applied for the determination of an unknown number of different 235U/238U isotopic signatures. The isotope ratios are again derived from the slopes of linear regression lines. In the ‘finite mixture model’, an algorithm applying fixed residual variances is used for clustering of the data points of interest and subsequent estimation of the slopes of the linear regression lines. The number of clusters, which represent the isotopic compositions, is estimated by the algorithm. Finite mixture models with components of the form (see Eqs. 1 and 2)
are considered as described in e.g. Leisch [32]. y is a dependent variable with conditional density h, x is a vector of independent variables, π k is the prior probability of component k, θ k is the component specific parameter vector for the density function f, and Ψ is the vector of all parameters.
In our case, we assume f to be a univariate normal density with component-specific mean β k x and non-component-specific fixed residual variance σ 2 for all values of k, so we have θ k = (β k , σ 2)′. We interpret x as 238U signal intensities, y as 235U signal intensities, K as the number of isotope ratios, β k as the isotope ratio of cluster k, σ 2 as the reproducibility of the measurement and π k as the percentage of data points belonging to cluster k. As we used blank corrected data, we forced the regressions through the origin, which kept the number of parameters in the model small. Using non-blank corrected data would need to include component-specific or non-component specific intercepts in the model, which would increase the complexity of the model significantly without additional benefit.
Parameter estimation (i.e. (β k , σ 2)) is done from N data points by maximising the log-likelihood function (see Eq. 3)
using an iterative expectation–maximization (EM) algorithm [39]. Determination of the number of isotope ratios is done by neglecting clusters below a certain threshold. In our case, clusters that contained less than 1 % of the data points in any iteration step were dropped. The final number of clusters is determined by the model fitting procedure. However, it is necessary to define an upper end of clusters as the EM algorithm can only reduce the number of clusters.
Computation was done in R [40], Version 2.15.2, using Grün and Leisch’s FlexMix package [41]. The raw data of 235U and 238U measurements were imported to the script. Blank correction and data selection by means of 3× SD was directly accomplished in R. An exemplary R script for the computation of multiple isotopic signatures is given in the Electronic Supplementary Material. It should be noted that the data given in this R script were simulated for demonstration purposes and are not corresponding to data recorded during LA-MC-ICPMS measurements.
Finally, the U isotope ratios were multiplied with the external correction factor \( \mathrm{C}{{\mathrm{F}}_{{{R_{{{}^{235}\mathrm{U}/{}^{238}\mathrm{U}}}}}}} \) to correct the isotope ratios for mass bias.
Data from 9073-01-B particle measurements (single isotopic composition) were used for the comparison of the ‘point-by-point’, the ‘integration’ and the ‘linear regression slope’ method. Data sets from 9073-01-B and CRM U010 were combined to one data set for the development of the ‘finite mixture model’. The NUSIMEP-7 test samples (multiple isotopic compositions) were evaluated by means of the ‘finite mixture model’ and compared to the commonly applied ‘point-by-point’ method.
Calculations of combined standard measurement uncertainties
Computation of expanded (k = 2) uncertainties (U) was performed according to ISO/GUM [42] and EURACHEM [43] guidelines with the GUM Workbench Pro software (Version 2.4, Metrodata GmbH, Weil am Rhein, Germany). The applied model equations are described as follows (i.e. Eqs. 4–6):
where \( {R_{{^{235}\mathrm{U}{/^{238}}\mathrm{U},\ \mathrm{Particle},\ \mathrm{final}}}} \) is the final 235U/238U isotope ratio of the measured particle; \( {R_{{^{235}\mathrm{U}{/^{238}}\mathrm{U},\ \mathrm{Particle},\ \mathrm{measured}}}} \) is the measured—blank corrected—235U/238U isotope ratio; and \( \mathrm{C}{{\mathrm{F}}_{{{R_{{^{235}\mathrm{U}{/^{238}}\mathrm{U}}}}}}} \) is the external correction factor, which was derived from the ratio of the certified (i.e. \( {R_{{^{235}\mathrm{U}{/^{238}}\mathrm{U},\ \mathrm{CR}{{\mathrm{M}}_{\mathrm{lq}}},\ \mathrm{certified}}}} \)) and the final, measured 235U/238U isotope ratio of the liquid CRM (i.e. \( {R_{{^{235}\mathrm{U}{/^{238}}\mathrm{U},\ \mathrm{CR}{{\mathrm{M}}_{\mathrm{lq}}},\ \mathrm{measured},\ \mathrm{final}}}} \)). \( {R_{{^{235}\mathrm{U}{/^{238}}\mathrm{U},\ \mathrm{CR}{{\mathrm{M}}_{\mathrm{lq}}},\ \mathrm{measured},\ \mathrm{final}}}} \) was expressed in a separate equation (i.e. Eq. 6) in order to not only account for the standard uncertainty (u) of the measurement of the liquid CRM but also for standard uncertainties resulting from blank contributions. \( {R_{{^{235}\mathrm{U}{/^{238}}\mathrm{U},\ \mathrm{CR}{{\mathrm{M}}_{\mathrm{lq}}},\ \mathrm{measured}}}} \) is the measured—blank corrected—235U/238U isotope ratio. Standard measurement uncertainties resulting from 235U and 238U, respectively, blank contributions from both LA and liquid measurements were accounted for by using so called δ-factors [44, 45] (i.e. \( {\delta_{{\mathrm{blank}\text{-}\mathrm{LA},\ {{}^{235}}\mathrm{U}}}} \), \( {\delta_{{\mathrm{blank}\text{-}\mathrm{LA}\text{,}{{}^{238}}\mathrm{U}}}} \), \( {\delta_{{\mathrm{blank}\text{-}\mathrm{lq}\text{,}{{}^{235}}\mathrm{U}}}} \) and \( {\delta_{{\mathrm{blank}\text{-}\mathrm{lq}\text{,}{{}^{238}}\mathrm{U}}}} \)).
The combined standard measurement uncertainties u c were multiplied with a coverage factor of 2 (i.e. k = 2) in order to obtain expanded uncertainties (U).
Results and discussion
Comparison of data evaluation methods for particles with single isotopic composition (CRM 9073-01-B)
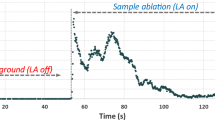
A typical transient signal record of single particle measurements by LA-MC-ICPMS is shown in Fig. 1.
The maximum 238U signal intensities of all analyzed 9073-01-B particles ranged from about 0.5 to 9.7 V. (Higher signal intensities led to a saturation of the detector and were not taken into account.) In Fig. 1, it can be seen that some peaks are exhibiting two or more peak maxima, which most likely result from adjacent particles entering the ICP almost simultaneously. To simplify matters for further evaluation, data points that belonged to one peak were regarded as signal intensities of one single particle. This simplification was considered as justified because the investigated test material has one certified U isotopic composition. Considering the ‘LRS’ method, two different evaluation approaches (i.e. y = ax and y = ax + b) were compared. In case of blank corrected data, the regression line was forced through the origin (i.e. y = ax), whereas the intercept (y = ax + b) was taken into account for non-blank corrected data. External correction was accomplished by means of IRMM-184. A summary of the different evaluation strategies is given in Table 3.
All evaluation strategies yield average 235U/238U isotope ratios that are, within their uncertainties, in accordance with the certified value. Although the ‘PBP’ method yielded the best precision and the smallest relative difference to the certified value in this study, the other strategies can be advantageous from a statistical point of view as they are taking weighted signal intensities into account. A comparison of the ‘LRS method’ with regard to its application to blank and non-blank corrected data yielded better precision and a smaller relative difference to the certified value for the blank corrected data, from which isotope ratios were computed by forcing the regression line through the origin. Moreover, forcing the regression line through the origin is regarded as advantageous, as in this case, it is ensured that high intensities are dominating the regression.
The uncertainties of single particle measurements with different maximum 238U signal intensities were calculated. Typically, apart from the ‘LRS’ (y = ax + b) method, the largest relative expanded uncertainties (RU) were observed for particles with the lowest signal intensities, whereas similar uncertainties were observed for particles with peak intensities higher than about 1.5 V. In Table 4, relative expanded uncertainties (RU) and relative contributions of input parameters are given for three single particles with different maximum 238U signal intensities.
The reproducibility (i.e. \( {R_{{^{235}\mathrm{U}{/^{238}}\mathrm{U},\ \mathrm{Particle},\ \mathrm{measured}}}} \)) was identified as the main contributor to the uncertainty. This is also in good accordance to previous work [6]. However, an exception from this was observed for the RU of the 235U/238U isotope ratio with the lowest maximal 238U signal intensity of the ‘PBP’ method. In this case, the main contribution resulted from the 235U LA blank (i.e. 62.2 %), followed by the reproducibility (i.e. 37.8 %). 235U blank contributions were also pronounced for the ‘integration’ and the ‘LRS’ (y = ax) method with respect to particles with the lowest peak intensities. The influence of the 235U LA blank is significantly reduced at higher signal intensities for the ‘integration’ and the ‘LRS’ (y = ax) method as compared to the ‘PBP’ method. This can be explained by the fact that in the ‘PBP’ method, high and low intensities are contributing equally to the isotope ratio.
‘Finite mixture model’
Evaluation methods taking weighted signal intensities into account are considered as data evaluation strategies of choice when dealing with short transient signals resulting from LA-MC-ICPMS analyses. Considering real safeguards samples, the typical situation is that one has to deal with an unknown number of particles that can differ in their isotopic signatures.
In Fig. 2, a typical transient signal recorded during raster scanning of a NUSIMEP-7 test sample is shown. LA of NUSIMEP-7 particles having an average diameter of 0.327 ± 0.139 μm yields very sharp signals with maximal 238U signal intensities below 1 V. In most cases, only one data point per particle was observed in the transient signal.
Thus, ‘integration’ and ‘PBP’ methods are becoming practically indistinguishable. The commonly applied approach for determining multiple isotopic signatures from transient signals is the ‘PBP’ method and plotting the isotope ratios in ascending order. In Fig. 3, the results of the ‘PBP’ method for the ‘double’ deposition NUSIMEP-7 test sample, which was expected to have two different isotopic compositions, is shown. As can be seen, two different major areas (i.e. two steps in the graph) of isotope ratios could be identified, indeed. In order to determine the isotopic compositions, the averages of the isotope ratios of these two areas were calculated. The data set was divided into two groups at the inflection points. Isotope ratios that were not within two times the standard deviation were not considered for calculating the average values. Isotope ratios that are between the two areas are typically considered as mixed ratios, whereas those at the lower and the upper end are typically regarded, dependent on their number, as other isotopic compositions or outliers resulting from the measurement. Based on the ‘PBP’ method, two main isotopic compositions (i.e. 0.0340(76) and 0.210(46)) were identified. A comparison with the certified isotopic compositions (Table 1) yields that one certified isotopic composition (i.e. 0.0090726(45)) of the original particles on the planchet was not identified. The reason is that the isotopic composition of the contamination, which occurred during sample handling, was dominant. The isotope ratios at the lower and upper end of the step-profile were regarded as outliers, as their number was rather small compared to the main isotopic compositions (one certified isotopic composition and the contamination). The drawbacks of this evaluation strategy for multiple isotopic signatures are evident: (1) low and high signal intensities are contributing equally to the isotope ratio, (2) the determination of the main compositions depends on the judgment of the analyst and (3) other isotopic compositions present may be hidden by the major constituents.
In order to improve data evaluation, we made use of the principle of the linear regression slope method. All signal intensities of interest are plotted in an x–y chart. Data from 9073-01-B and CRM U010 particle measurements were combined for a test data set in order to simulate a sample with particles of different known isotopic compositions. In Fig. 4a, the scatter plot of this test data set is shown.
Determination of two different 235U/238U isotope ratios by means of the slopes of linear regression lines applying the ‘finite mixture model’. a Scatter plot of a data set of 9073-01-B and CRM U010 particle measurements that were combined to one test data set. b Application of the ‘finite mixture model’ to the test data set: the circles and the triangles are representing the two clusters (i.e. two isotopic signatures) that are distinguishable. The isotopic signatures are determined by means of the slope of the linear regression lines. The slopes that are stated in the legend are not externally corrected. Outliers are marked by grey-coloured circles
At this step, no differentiation between the data points of different isotopic compositions can be accomplished. A ‘finite mixture model’ was applied for the deconvolution of this data set to determine the different 235U/238U isotopic signatures. The data points are clustered, which is demonstrated by the two different symbols (i.e. circle and triangle; Fig. 4b). Finally, the slopes of the linear relationships and the respective uncertainties of the clustered data points are estimated by the model [32, 41]. All selected data points are contributing to each linear fit, and the regression lines are forced through the origin. Higher signal intensities have a larger leverage effect on the slope than lower ones, which is given by the model. In an additional step, the isotope ratios that are derived from the slope of the regression lines have to be corrected by means of the external correction factor \( \mathrm{C}{{\mathrm{F}}_{{{R_{{^{235}\mathrm{U}{/^{238}}\mathrm{U}}}}}}} \) (equal to the other data evaluation strategies). In the case of the test data set, external correction was accomplished by means of IRMM-184.
Fitting mixture models by the EM algorithm is known to be sensitive to outliers in the data (see Fig 4b). Outlier elimination can be accomplished by using a robust version of the EM algorithm, which includes a background noise component with a very large variance collecting all outliers [46]. However, in our case, it is important to identify outliers, as these data points might indicate an additional isotopic composition. This was accomplished by fixing the variances of all components to be the same. Moreover, this reduced the number of the estimated parameters.
In addition, it is possible to easily trace back single data points that are far off from the computed linear regression lines to the raw data set by using R. The test data set consists of two certified 235U/238U isotopic signatures. Data points that are outside the uncertainty of the computed isotopic compositions (i.e. slopes) can be excluded, and the model is recalculated. Recalculation and outlier detection are performed according to an iterative approach. The principle of outlier detection is discussed for two data points marked in Fig. 4b. The outliers resulted from different decay times of the two Faraday detectors. The transient signals revealed that the Faraday detector L1 (238U) had been saturated just before the signal of the outlier was recorded. Therefore, these data points were regarded as measurement artefacts and excluded from the regression. Outlier elimination resulted in smaller relative differences to the certified values with regard to the determined isotopic signatures (from 0.2 % to −0.03 % for 9073-01-B and from 0.6 % to 0.5 % for U010) and reduced the uncertainties of the slope (from 14 % to 10 % for 9073-01-B and from 10 % to 7 % for U010). Expanded uncertainties (Eqs. 4–6) of 19 % (k = 2) and 14 % (k = 2) were computed for the 235U/238U isotopic signature of 9073-01-B (i.e. 0.00725) and U010 (i.e. 0.01019), respectively. The major contributor to the uncertainty is the uncertainty of the slope which is determined by the fixed residual variances of the model.
Application of the ‘finite mixture model’ to NUSIMEP-7 interlaboratory comparison test samples
The ‘finite mixture model’ was applied to the 235U and 238U measurement data of the NUSIMEP-7 interlaboratory comparison test samples in order to investigate its applicability for the determination of different unknown U isotopic compositions. The clusters and linear regression lines that were computed for both the ‘single’ and the ‘double’ deposition samples by the ‘finite mixture model’ are shown in Fig. 5.
Application of the finite mixture model to the blank corrected 235U and 238U measurement data of the NUSIMEP-7 interlaboratory comparison test samples: (a) NUSIMEP-7 ‘single’ deposition; (b) NUSIMEP-7 ‘double’ deposition. A contamination during sample handling with a 235U enrichment of about 21 % was detected on both planchets
The ‘finite mixture model’ yielded 235U/238U isotopic compositions that were, within their uncertainties, in good agreement with the certified values (Table 5). Nonetheless, the uncertainties were larger compared to uncertainties of SIMS measurements in this ILC [38]. SIMS is considered as mainstay technique for particle analysis. Both test samples had been contaminated with a 235U enrichment of about 21 %. Thus, one additional isotopic composition was determined for both the ‘single’ and ‘double’ deposition test samples. The additional isotopic composition of the contaminant is in agreement between the two test samples indicating the same source of contamination.
Moreover, z and zeta scores were calculated in order to assess the performance of LA-MC-ICPMS measurements, using the ‘finite mixture model’ for data evaluation, with respect to the stringent performance criteria of the NUSIMEP-7 ILC for the major U (235U/238U) isotope ratio [38]. As can be seen in Table 5, regarding the z scores, some measurements could not be performed in accordance with requirements considered as good practice for IAEA Network Analytical Laboratories (NWAL) (i.e. |score| ≤ 2). According to the NUSIMEP-7 report [38], less than 50 % of the participants reported satisfactory results (i.e. 47 % for the ‘single’ deposition, 41 % for the first enrichment and 35 % for the second enrichment of the ‘double’ deposition). In case of the second enrichment, satisfactory results were only achieved by large geometry-secondary ion mass spectrometry (LG-SIMS), nanoSIMS and secondary electron microscope-thermal ionization mass spectrometry (SEM-TIMS) measurements [38]. Worth mentioning is that the organizers stated in their NUSIMEP-7 report [38] that questionable results would still have been satisfactory when applying the less stringent performance criteria from NUSIMEP-6, the previous ILC [38].
Regarding the zeta scores, satisfactory results (i.e. |score| ≤ 2) were achieved for all isotopic compositions, which indicates that the estimate of the uncertainties are consistent with the deviations from the reference value [38].
In the ‘finite mixture model’, the whole computation—blank correction, data selection by means of 3× SD, determination of the number of clusters, computation of the slope and of its uncertainty—of the data set having about 50,000 data points (including the blank measured in between the particle signals) is accomplished automatically by the applied algorithm. Thus, information about the isotopic composition is readily available. Moreover, data evaluation is not influenced by the analyst. In addition, the application of the ‘finite mixture model’ to the NUSIMEP-7 ‘double’ deposition sample (Table 5) demonstrated the model’s strength for the determination of an accurate isotopic signature of a rather small population (i.e. 29 data points) present besides two larger ones (i.e. 122 and 633 data points).
Conclusions
In this study, four different data treatment strategies for the computation of 235U/238U isotope ratios of single particles from transient signals were evaluated. Generally, strategies in which higher signal intensities assume greater weight in the isotope ratio calculation (‘integration’ and ‘linear regression slope’ method) are regarded as advantageous from a statistical point of view. However, these methods can only be applied if individual particles can be analyzed and if the resulting peak consists of multiple data points. Considering real safeguards samples, an individual selection of the particles to be analyzed is often not feasible using LA-MC-ICPMS without pre-selection by means of e.g. fission track or scanning electron microscopy. Hence, the resulting peak may consist of data points from several particles having different isotopic compositions. Moreover, depending on the size of the particles, only one data point may be available for subsequent data treatment. The commonly applied ‘point-by-point’ method bears the danger to overlook isotopic compositions of particles being underrepresented. A ‘finite mixture model’ based on linear regression enables to identify an unknown number of entities of different isotopic composition, thus providing a significant contribution to fields dealing with multiple isotope ratios in mixed samples.
Even though in this study only results for the major U isotope ratio (235U/238U) were shown, the model is equally applicable for the determination of the minor isotope ratios (i.e. 234U/238U and 236U/238U). These ratios are equally important in nuclear safeguards and forensics for verifying the absence of undeclared nuclear activities. The applicability of LA-MC-ICPMS for the determination of the minor U isotopes was demonstrated in a recent study [6], as well as within the NUSIMEP-7 ILC [38].
References
Axelsson A, Fischer DM, Pénkin MV (2009) Use of data from environmental sampling for IAEA safeguards. Case study: uranium with near-natural 235U abundance. J Radioanal Nucl Chem 282:725–729
Mayer K, Wallenius M, Fanghänel T (2007) Nuclear forensic science—from cradle to maturity. J Alloys Compd 444–445:50–56
Donohue DL (1998) Strengthening IAEA safeguards through environmental sampling and analysis. J Alloys Compd 271–273:11–18
Donohue DL (2002) Peer reviewed: strengthened nuclear safeguards. Anal Chem 74:28 A–35 A
Zendel M, Donohue DL, Kuhn E, Deron S, Bíró T (2011) In: Vértes A, Nagy S, Klencsár Z, Lovas RG, Rösch F (eds) Nuclear safeguards verification measurement techniques. Springer Science + Business Media B.V, Dordrecht
Kappel S, Boulyga SF, Prohaska T (2012) Direct uranium isotope ratio analysis of single micrometer-sized glass particles. J Environ Radioactiv 113:8–15
Varga Z (2008) Application of laser ablation inductively coupled plasma mass spectrometry for the isotopic analysis of single uranium particles. Anal Chim Acta 625:1–7
Lloyd NS, Parrish RR, Horstwood MSA, Chenery SRN (2009) Precise and accurate isotopic analysis of microscopic uranium-oxide grains using LA-MC-ICP-MS. J Anal At Spectrom 24:752–758
Boulyga SF, Prohaska T (2008) Determining the isotopic compositions of uranium and fission products in radioactive environmental microsamples using laser ablation ICP-MS with multiple ion counters. Anal Bioanal Chem 390:531–539
Pointurier F, Pottin AC, Hubert A (2011) Application of nanosecond-UV laser ablation-inductively coupled plasma mass spectrometry for the isotopic analysis of single submicrometer-size uranium particles. Anal Chem 83:7841–7848
Aregbe Y, Prohaska T, Stefanka Z, Széles É, Hubert A, Boulyga S (2011) Report on the workshop on direct analysis of solid samples using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS)—organised by the ESARDA working group on standards and techniques for destructive analysis (WG DA). Esarda Bulletin 46:136–145
Günther-Leopold I, Wernli B, Kopajtic Z, Günther D (2004) Measurement of isotope ratios on transient signals by MC-ICP–MS. Anal Bioanal Chem 378:241–249
Epov VN, Berail S, Jimenez-Moreno M, Perrot V, Pecheyran C, Amouroux D, Donard OFX (2010) Approach to measure isotopic ratios in species using multicollector-ICPMS coupled with chromatography. Anal Chem 82:5652–5662
Krupp EM, Donard OFX (2005) Isotope ratios on transient signals with GC-MC-ICP-MS. Int J Mass Spectrom 242:233–242
Krupp EM, Pécheyran C, Pinaly H, Motelica-Heino M, Koller D, Young SMM, Brenner IB, Donard OFX (2001) Isotopic precision for a lead species (PbEt4) using capillary gas chromatography coupled to inductively coupled plasma-multicollector mass spectrometry. Spectrochim Acta B 56:1233–1240
Galler P, Limbeck A, Boulyga SF, Stingeder G, Hirata T, Prohaska T (2007) Development of an on-line flow injection sr/matrix separation method for accurate, high-throughput determination of sr isotope ratios by multiple collector-inductively coupled plasma-mass spectrometry. Anal Chem 79:5023–5029
Evans RD, Hintelmann H, Dillon PJ (2001) Measurement of high precision isotope ratios for mercury from coals using transient signals. J Anal At Spectrom 16:1064–1069
Xie Q, Lu S, Evans D, Dillon P, Hintelmann H (2005) High precision Hg isotope analysis of environmental samples using gold trap-MC-ICP-MS. J Anal At Spectrom 20:515–522
Hirata T, Yamaguchi T (1999) Isotopic analysis of zirconium using enhanced sensitivity-laser ablation-multiple collector-inductively coupled plasma mass spectrometry. J Anal At Spectrom 14:1455–1459
Rodríguez-González P, Epov VN, Pecheyran C, Amouroux D, Donard OFX (2011) Species-specific stable isotope analysis by the hyphenation of chromatographic techniques with MC-ICPMS. Mass Spectrom Rev 31:504–521
Skoog DA, Leary JJ (1992) Principles of instrumental analysis, 4th edn. Saunders College Publishing, Philadelphia
Cottle JM, Horstwood MSA, Parrish RR (2009) A new approach to single shot laser ablation analysis and its application to in situ Pb/U geochronology. J Anal At Spectrom 24:1355–1363
Fricker MB, Kutscher D, Aeschlimann B, Frommer J, Dietiker R, Bettmer J, Günther D (2011) High spatial resolution trace element analysis by LA-ICP-MS using a novel ablation cell for multiple or large samples. Int J Mass Spectrom 307:39–45
Dzurko M, Foucher D, Hintelmann H (2009) Determination of compound-specific Hg isotope ratios from transient signals using gas chromatography coupled to multicollector inductively coupled plasma mass spectrometry (MC-ICP/MS). Anal Bioanal Chem 393:345–355
Wehmeier S, Ellam R, Feldmann J (2003) Isotope ratio determination of antimony from the transient signal of trimethylstibine by GC-MC-ICP-MS and GC-ICP-TOF-MS. J Anal At Spectrom 18:1001–1007
Krupp E, Pécheyran C, Meffan-Main S, Donard OX (2004) Precise isotope-ratio determination by CGC hyphenated to ICP–MCMS for speciation of trace amounts of gaseous sulfur, with SF6 as example compound. Anal Bioanal Chem 378:250–255
Günther-Leopold I, Waldis JK, Wernli B, Kopajtic Z (2005) Measurement of plutonium isotope ratios in nuclear fuel samples by HPLC-MC-ICP-MS. Int J Mass Spectrom 242:197–202
Hirata T, Hayano Y, Ohno T (2003) Improvements in precision of isotopic ratio measurements using laser ablation-multiple collector-ICP-mass spectrometry: reduction of changes in measured isotopic ratios. J Anal At Spectrom 18:1283–1288
Pettke T, Oberli F, Audetat A, Wiechert U, Harris CR, Heinrich CA (2011) Quantification of transient signals in multiple collector inductively coupled plasma mass spectrometry: accurate lead isotope ratio determination by laser ablation of individual fluid inclusions. J Anal At Spectrom 26:475–492
Fietzke J, Liebetrau V, Günther D, Gurs K, Hametner K, Zumholz K, Hansteen TH, Eisenhauer A (2008) An alternative data acquisition and evaluation strategy for improved isotope ratio precision using LA-MC-ICP-MS applied to stable and radiogenic strontium isotopes in carbonates. J Anal At Spectrom 23:955–961
Rodríguez-Castrillón JA, García-Ruiz S, Moldovan M, García Alonso JI (2012) Multiple linear regression and on-line ion exchange chromatography for alternative Rb-Sr and Nd-Sm MC-ICP-MS isotopic measurements. J Anal At Spectrom 27:611–618
Leisch F (2004) FlexMix: a general framework for finite mixture models and latent class regression in R. J Stat Softw 11:1–18
IRMM (2005) Certificate isotopic reference material IRMM-184. http://irmm.jrc.ec.europa.eu/reference_materials_catalogue/catalogue/IRMM_certificates_and_reports/irmm-184_cert.pdf. Accessed 8 November 2012
New Brunswick Laboratory–US Department of Energy (2008) Certificate of analysis CRM U030-A—uranium isotopic standard. http://www.nbl.doe.gov/docs/pdf/CRM_U030-A_10_milligram_Sample_Size_March_2008.pdf. Accessed 8 November 2012
New Brunswick Laboratory–US Department of Energy (2008) Certificate of analysis CRM U500—uranium isotopic standard. http://www.nbl.doe.gov/docs/pdf/CRM_U500_10_milligram_Sample_Size_March_2008.pdf. Accessed 8 November 2012
IRMM (1997) Certificate of isotopic composition, sample identification: 9073-01-B
New Brunswick Laboratory–US Department of Energy (2008) Certificate of analysis CRM U010—uranium isotopic standard. http://www.nbl.doe.gov/docs/pdf/CRM_U010_5_milligram_Sample_Size_March_2008.pdf. Accessed 8 November 2012
Truyens J, Stefaniak E, Mialle S, Aregbe Y (2011) NUSIMEP-7: uranium isotope amount ratios in uranium particles. Interlaboratory Comparison Report. http://irmm.jrc.ec.europa.eu/interlaboratory_comparisons/nusimep/Nusimep-7/Documents/eur_25179_en%20_nusimep_7_report_to_participants.pdf. Accessed 28 June 2012
Dempster AP, Laird NM, Rubin DB (1977) Maximum likelihood from incomplete data via the EM algorithm. J Roy Stat Soc B Met 39:1–38
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN: 3-900051-07-0. http://www.R-project.org/
Grün B, Leisch F (2008) FlexMix version 2: finite mixtures with concomitant variables and varying and constant parameters. J Stat Softw 28:1–35
ISO/IEC Guide 98-3:2008. Uncertainty of measurement—part 3: guide to the expression of uncertainty in measurement (GUM: 1995)
EURACHEM/CITAC Guide CG 4. Quantifying uncertainty in analytical measurement (2000), 2nd edn
Bürger S, Essex RM, Mathew KJ, Richter S, Thomas RB (2010) Implementation of Guide to the expression of uncertainty in measurement (GUM) to multi-collector TIMS uranium isotope ratio metrology. Int J Mass Spectrom 294:65–76
European Co-operation for Accreditation. Expression of the uncertainty of measurement in calibration. EA-4/02 M:1999
Leisch F (2008) Modelling background noise in finite mixtures of generalized linear regression models. Compstat 2008:385–396
Acknowledgements
The Austrian Science Fund FWF (START project 267-N11) and the International Atomic Energy Agency are highly acknowledged for financial support. The authors also thank Thomas Konegger from the Institute of Chemical Technologies and Analytics at the Vienna University of Technology for performing the scanning electron microscope analyses of the particles. We also thank Stefan Bürger for the fruitful discussions about uncertainty propagations of U isotope ratio measurements. We thank Andreas Zitek for supporting this work with regard to the deconvolution of multiple isotope ratios in mixed samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Isotope Ratio Measurements: New Developments and Applications with guest editors Klaus G. Heumann and Torsten C. Schmidt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 62.4 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kappel, S., Boulyga, S.F., Dorta, L. et al. Evaluation strategies for isotope ratio measurements of single particles by LA-MC-ICPMS. Anal Bioanal Chem 405, 2943–2955 (2013). https://doi.org/10.1007/s00216-012-6674-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6674-3