Abstract
Background and Objective
Oxycodone, a semisynthetic thebaine derivative µ-opioid (MOP) receptor agonist, is effective for treating moderate and severe pain in different clinical conditions. The pharmacokinetics of intravenous oxycodone in the obese population has not been studied. This study aims to characterize the pharmacokinetic profile of oxycodone after intravenous administration and to simulate an appropriate dosage for analgesic efficacy in obese patients.
Methods
We recruited 33 (age range from 21 to 72 years) adult patients with a body mass index of 30 kg/m2 and above, who were scheduled for non-cardiac surgeries. Intravenous oxycodone was administered after induction of general anesthesia and blood samples were collected up to 24 h after oxycodone administration. Plasma concentrations of oxycodone were assayed using liquid chromatography-tandem mass spectrometry and 253 concentration–time points were used for pharmacokinetic analysis using nonlinear mixed-effects modeling.
Results
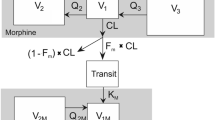
Intravenous oxycodone pharmacokinetics were well described by a two-compartment open model. The estimated total clearance and central volume of distribution of oxycodone are 28.5 l/h per 70 kg and 56.4 l per 70 kg, respectively. Total body weight was identified as a significant covariate of the clearance and central volume of distribution. Dosing simulations based on the final model demonstrate that a starting dose of 0.10 mg/kg of intravenous oxycodone is adequate to achieve a target plasma concentration and repeated doses of 0.02 mg/kg may be administered at 1.5-h intervals to maintain a plasma concentration within an effective analgesic range.
Conclusions
A population pharmacokinetic model using total body weight as a covariate supports the administration of 0.10 mg/kg of intravenous oxycodone as a starting dose and repeated doses of 0.02 mg/kg at 1.5-h intervals to maintain targeted plasma concentrations for analgesia in the obese adult population.



Similar content being viewed by others
References
Poyhia R. Opioids in anaesthesia: a questionnaire survey in Finland. Eur J Anaesthesiol. 1994;11(3):221–30.
Kalso E, Poyhia R, Onnela P, Linko K, Tigerstedt I, Tammisto T. Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand. 1991;35(7):642–6.
Ennis M, Schneider C, Nehring E, Lorenz W. Histamine release induced by opioid analgesics: a comparative study using porcine mast cells. Agents Actions. 1991;33(1–2):20–2.
Eisenach JC, Carpenter R, Curry R. Analgesia from a peripherally active kappa-opioid receptor agonist in patients with chronic pancreatitis. Pain. 2003;101(1–2):89–95.
Olesen AE, Upton R, Foster DJ, Staahl C, Christrup LL, Arendt-Nielsen L, Drewes AM. A pharmacokinetic and pharmacodynamic study of oral oxycodone in a human experimental pain model of hyperalgesia. Clin Pharmacokinet. 2010;49(12):817–27.
Poulsen KK, Andersen SE, Moreno SI, Glintborg D, Thirstrup S, Aagaard L. General practitioners’ and hospital physicians’ preference for morphine or oxycodone as first-time choice for a strong opioid: a National Register-based study. Basic Clin Pharmacol Toxicol. 2013;112(2):110–5.
Roxburgh A, Bruno R, Larance B, Burns L. Prescription of opioid analgesics and related harms in Australia. Med J Aust. 2011;195(5):280–4.
Kinnunen M, Piirainen P, Kokki H, Lammi P, Kokki M. Updated clinical pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacokinet. 2019;58(6):705–25.
Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304.
Poyhia R, Seppala T, Olkkola KT, Kalso E. The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol. 1992;33(6):617–21.
Kirvela M, Lindgren L, Seppala T, Olkkola KT. The pharmacokinetics of oxycodone in uremic patients undergoing renal transplantation. J Clin Anesth. 1996;8(1):13–8.
Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther. 2006;79(5):461–79.
Lalovic B, Phillips B, Risler LL, Howald W, Shen DD. Quantitative contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab Dispos. 2004;32(4):447–54.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Kokubun H, Yoshimoto T, Hojo M, Fukumura K, Matoba M. Pharmacokinetics of oxycodone after intravenous and subcutaneous administration in Japanese patients with cancer pain. J Pain Palliat Care Pharmacother. 2014;28(4):338–50.
Poyhia R, Olkkola KT, Seppala T, Kalso E. The pharmacokinetics of oxycodone after intravenous injection in adults. Br J Clin Pharmacol. 1991;32(4):516–8.
Choi BM, Lee YH, An SM, Lee SH, Lee EK, Noh GJ. Population pharmacokinetics and analgesic potency of oxycodone. Br J Clin Pharmacol. 2017;83(2):314–25.
Saari TI, Ihmsen H, Neuvonen PJ, Olkkola KT, Schwilden H. Oxycodone clearance is markedly reduced with advancing age: a population pharmacokinetic study. Br J Anaesth. 2012;108(3):491–8.
El-Tahtawy A, Kokki H, Reidenberg BE. Population pharmacokinetics of oxycodone in children 6 months to 7 years old. J Clin Pharmacol. 2006;46(4):433–42.
Liukas A, Kuusniemi K, Aantaa R, Virolainen P, Neuvonen M, Neuvonen PJ, Olkkola KT. Elimination of intravenous oxycodone in the elderly: a pharmacokinetic study in postoperative orthopaedic patients of different age groups. Drugs Aging. 2011;28(1):41–50.
Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91.
OxyNorm (Solution for injection or infusion). Available from https://www.medicines.org.uk/emc/product/6795/smpc. Accessed 15 Aug 2022.
Kokki M, Broms S, Eskelinen M, Rasanen I, Ojanperä I, Kokki H. Analgesic concentrations of oxycodone–A prospective clinical PK/PD study in patients with laparoscopic cholecystectomy. Basic Clin Pharmacol Toxicol. 2012;110(5):469–75.
Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed. 2011;101(1):72–9.
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051–65.
Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology C. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–72.
Morse JD, Hannam JA, Anderson BJ, Kokki H, Kokki M. Oxycodone target concentration dosing for acute pain in children. Paediatr Anaesth. 2021;31(12):1325–31.
Pesonen A, Suojaranta-Ylinen R, Hammaren E, Tarkkila P, Seppala T, Rosenberg PH. Comparison of effects and plasma concentrations of opioids between elderly and middle-aged patients after cardiac surgery. Acta Anaesthesiol Scand. 2009;53(1):101–8.
Schulz M, Iwersen-Bergmann S, Andresen H, Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit Care. 2012;16(4):R136.
Nieminen TH, Hagelberg NM, Saari TI, Pertovaara A, Neuvonen M, Laine K, Neuvonen PJ, Olkkola KT. Rifampin greatly reduces the plasma concentrations of intravenous and oral oxycodone. Anesthesiology. 2009;110(6):1371–8.
Mandema JW, Kaiko RF, Oshlack B, Reder RF, Stanski DR. Characterization and validation of a pharmacokinetic model for controlled-release oxycodone. Br J Clin Pharmacol. 1996;42(6):747–56.
Guzzaloni G, Grugni G, Minocci A, Moro D, Morabito F. Liver steatosis in juvenile obesity: correlations with lipid profile, hepatic biochemical parameters and glycemic and insulinemic responses to an oral glucose tolerance test. Int J Obes Relat Metab Disord. 2000;24(6):772–6.
Wree A, Kahraman A, Gerken G, Canbay A. Obesity affects the liver - the link between adipocytes and hepatocytes. Digestion. 2011;83(1–2):124–33.
Villesen HH, Banning AM, Petersen RH, Weinelt S, Poulsen JB, Hansen SH, Christrup LL. Pharmacokinetics of morphine and oxycodone following intravenous administration in elderly patients. Ther Clin Risk Manag. 2007;3(5):961–7.
Klimas R, Witticke D, El Fallah S, Mikus G. Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration. Expert Opin Drug Metab Toxicol. 2013;9(5):517–28.
Acknowledgements
The authors are grateful to the staff involved in the study. Mr. Mohd Shafiq for helping in the storage of plasma samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by Sook Hui Chaw and Yoke Lin Lo. The first draft of the manuscript was written by Sook Hui Chaw and all authors commented on the manuscript. The final version of the manuscript was read and approved by all authors.
Funding
This study received funding from Institut Pengurusan dan Pemantauan Penyelidikan, Universiti Malaya, Bantuan Kecil Penyelidikan (BKP) BK031-2016.
Conflict of interest
Sook Hui Chaw, Yoke Lin Lo, Li Ling Yeap, Didi Erwandi Bin Mohamad Haron, and Ina Ismiarti Shariffuddin declare that they have no conflicts of interest.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The model code can be obtained from the corresponding author on reasonable request.
Ethics approval
This study was performed in line with the principles of the 1964 Declaration of Helsinki and its later amendments. Approval was granted by the UMMC Medical Ethics and Research Committee (MERC number: 20162-2143).
Consent to participate
Informed consent was obtained from all participants included in the study.
Cosent for publication
Not applicable.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaw, S.H., Lo, Y.L., Yeap, L.L. et al. Population Pharmacokinetics and Dosing Simulations of Intravenous Oxycodone for Perioperative Pain Relief in Adult Surgical Patients with Obesity. Eur J Drug Metab Pharmacokinet 48, 11–21 (2023). https://doi.org/10.1007/s13318-022-00795-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00795-4