Abstract
Background and Objective
Morphine is a standard analgesic drug for postoperative pain therapy. This study aimed to evaluate the pharmacokinetics of morphine and its active metabolite morphine-6-glucuronide (M6G) in cardiac surgery patients during postoperative analgesia.
Methods
Twenty-five adult patients undergoing cardiac surgery received postoperative pain therapy by patient-controlled analgesia with intravenous bolus doses of morphine. Plasma concentrations of morphine and M6G were determined from arterial samples. Population pharmacokinetic parameters were estimated using nonlinear mixed-effects modeling.
Results
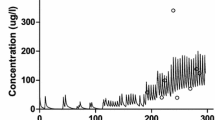
Data from twenty-one patients (aged 44–79 years) were analyzed. Pharmacokinetics were best described by a three-compartment model for morphine and a two-compartment model for M6G, linked by a transit compartment. Mean (±SD) population estimates for morphine were: clearance (CL) = 1.35±0.40 L/min, central volume of distribution (V1) = 8.1±2.2 L, steady-state volume of distribution (Vss) = 207±83 L, terminal elimination half-life (T1/2γ) = 177±50 min. Clearance of morphine was proportional to cardiac output. Mean (±SD) population estimates for M6G were: CL = 0.098±0.037 L/min, V1 = 5.5±0.8 L, Vss = 15.8±0.8 L, T1/2β = 227±74 min. The time to peak concentration of M6G after a bolus dose of morphine was 53±20 min. Clearance of M6G was proportional to estimated glomerular filtration rate.
Conclusions
The pharmacokinetics of morphine and M6G in pain therapy of cardiac surgery patients could be well described by standard compartmental models. Cardiac output was identified as a significant covariate for morphine clearance, whereas renal function was identified as the most significant covariate for clearance of M6G. These effects should be particularly considered if morphine is administered as a continuous infusion. The developed pharmacokinetic model also enables patient-controlled target-controlled infusion for pain therapy with morphine.
Trial Registration
Clinical Trials NCT02483221 (June 26, 2015).









Similar content being viewed by others
Availability of Data and Material
Data are available on request from the authors
References
Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2006; 4: CD003348.
Sverrisdottir E, Lund TM, Olesen AE, Drewes AM, Christrup LL, Kreilgaard M. A review of morphine and morphine-6-glucuronide’s pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur J Pharm Sci. 2015;74:45–62.
Penson RT, Joel SP, Bakhshi K, Clark SJ, Langford RM, Slevin ML. Randomized placebo-controlled trial of the activity of the morphine glucuronides. Clin Pharmacol Ther. 2000;68:667–76.
van Dorp EL, Morariu A, Dahan A. Morphine-6-glucuronide: potency and safety compared with morphine. Expert Opin Pharmacother. 2008;9:1955–61.
Lötsch J, Skarke C, Schmidt H, Liefhold J, Geisslinger G. Pharmacokinetic modeling to predict morphine and morphine-6-glucuronide plasma concentrations in healthy young volunteers. Clin Pharmacol Ther. 2002;72:151–62.
Lötsch J, Weiss M, Kobal G, Geisslinger G. Pharmacokinetics of morphine-6-glucuronide and its formation from morphine after intravenous administration. Clin Pharmacol Ther. 1998;63:629–39.
Mazoit JX, Butscher K, Samii K. Morphine in postoperative patients: pharmacokinetics and pharmacodynamics of metabolites. Anesth Analg. 2007;105:70–8.
Meineke I, Freudenthaler S, Hofmann U, Schaeffeler E, Mikus G, Schwab M, et al. Pharmacokinetic modelling of morphine, morphine-3-glucuronide and morphine-6-glucuronide in plasma and cerebrospinal fluid of neurosurgical patients after short-term infusion of morphine. Br J Clin Pharmacol. 2002;54:592–603.
Crotty B, Watson KJ, Desmond PV, Mashford ML, Wood LJ, Colman J, et al. Hepatic extraction of morphine is impaired in cirrhosis. Eur J Clin Pharmacol. 1989;36:501–6.
Stanski DR, Greenblatt DJ, Lowenstein E. Kinetics of intravenous and intramuscular morphine. Clin Pharmacol Ther. 1978;24:52–9.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–95.
Iacoviello M, Leone M, Antoncecchi V, Ciccone MM. Evaluation of chronic kidney disease in chronic heart failure: from biomarkers to arterial renal resistances. World J Clin Cases. 2015;3:10–9.
Hasselstrom J, Sawe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24:344–54.
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65.
DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57.
Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology. 1992;76:334–41.
Dahan A, Romberg R, Teppema L, Sarton E, Bijl H, Olofsen E. Simultaneous measurement and integrated analysis of analgesia and respiration after an intravenous morphine infusion. Anesthesiology. 2004;101:1201–9.
Romberg R, Olofsen E, Sarton E, den Hartigh J, Taschner PE, Dahan A. Pharmacokinetic-pharmacodynamic modeling of morphine-6-glucuronide-induced analgesia in healthy volunteers: absence of sex differences. Anesthesiology. 2004;100:120–33.
Hand CW, Moore RA, McQuay HJ, Allen MC, Sear JW. Analysis of morphine and its major metabolites by differential radioimmunoassay. Ann Clin Biochem. 1987;24(Pt 2):153–60.
de Hoogd S, Valitalo PAJ, Dahan A, van Kralingen S, Coughtrie MMW, van Dongen EPA, et al. Influence of morbid obesity on the pharmacokinetics of morphine, morphine-3-glucuronide, and morphine-6-glucuronide. Clin Pharmacokinet. 2017;56:1577–87.
Villesen HH, Banning AM, Petersen RH, Weinelt S, Poulsen JB, Hansen SH, et al. Pharmacokinetics of morphine and oxycodone following intravenous administration in elderly patients. Ther Clin Risk Manag. 2007;3:961–7.
Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93:1245–54.
Dahlstrom B, Tamsen A, Paalzow L, Hartvig P. Patient-controlled analgesic therapy, Part IV: pharmacokinetics and analgesic plasma concentrations of morphine. Clin Pharmacokinet. 1982;7:266–79.
Hanna MH, D’Costa F, Peat SJ, Fung C, Venkat N, Zilkha TR, et al. Morphine-6-glucuronide disposition in renal impairment. Br J Anaesth. 1993;70:511–4.
Osborne R, Joel S, Grebenik K, Trew D, Slevin M. The pharmacokinetics of morphine and morphine glucuronides in kidney failure. Clin Pharmacol Ther. 1993;54:158–67.
Sear JW, Hand CW, Moore RA, McQuay HJ. Studies on morphine disposition: influence of renal failure on the kinetics of morphine and its metabolites. Br J Anaesth. 1989;62:28–32.
Skarke C, Darimont J, Schmidt H, Geisslinger G, Lötsch J. Analgesic effects of morphine and morphine-6-glucuronide in a transcutaneous electrical pain model in healthy volunteers. Clin Pharmacol Ther. 2003;73:107–21.
Acknowledgements
The authors thank Rainer Knoll, Dipl. Bioingenieur (Department of Anesthesiology, University of Erlangen-Nürnberg, Erlangen, Germany) for conducting the drug analysis, and Gabriele Göhring-Waldeck, Laboratory Technician (Department of Anesthesiology, University of Erlangen-Nürnberg, Erlangen, Germany) for help in patient recruitment and study organization.
Author information
Authors and Affiliations
Contributions
Harald Ihmsen: Data acquisition, analysis and interpretation, drafting of the manuscript. Jürgen Schüttler: Analysis and interpretation of the data. Christian Jeleazcov: Study conception and design, acquisition, analysis and interpretation of the data. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This work was supported by a grant from the German Federal Ministry of Education and Research, Berlin, Germany (Bundesministerium für Bildung und Forschung, grant no: FKZ 13EX1015B).
Conflict of Interest
Harald Ihmsen, Jürgen Schüttler and Christian Jeleazcov declare that they have no conflict of interest.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval was obtained from the Institutional Ethical Committee (Ethikkommission der Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany).
Consent
Written informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ihmsen, H., Schüttler, J. & Jeleazcov, C. Pharmacokinetics of Morphine and Morphine-6-Glucuronide During Postoperative Pain Therapy in Cardiac Surgery Patients. Eur J Drug Metab Pharmacokinet 46, 249–263 (2021). https://doi.org/10.1007/s13318-020-00663-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-020-00663-z