Abstract
Background and Objective
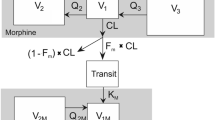
We recently developed a new population pharmacokinetic model for hydromorphone in patients including age and bodyweight as covariates. The aim of the present study was to evaluate prospectively the predictive performance of this new model during postoperative pain therapy.
Methods
This was a prospective, single-blinded, randomized, single-center study with two parallel arms. Fifty patients aged 40–85 years undergoing cardiac surgery involving thoracotomy were enrolled. Hydromorphone was administered postoperatively on the intensive care unit as target controlled infusion (TCI) for patient controlled analgesia (TCI-PCA) using the new pharmacokinetic model, or as conventional patient controlled analgesia (PCA). Arterial blood samples were taken for measurement of the hydromorphone plasma concentration. The predictive performance of the pharmacokinetic model was assessed by the median performance error (MDPE), the median absolute performance error (MDAPE), wobble and divergence. For comparison, the performance indices were also determined for three older models from the literature.
Results
903 plasma concentrations of 41 patients were analyzed. The mean values (95 % CI) of MDPE, MDAPE, wobble and divergence for the new pharmacokinetic model were 11.2 % (3.9 to 18.7 %), 28.5 % (23.9 to 33.0 %), 21.4 % (18.0 to 24.9 %) and −1.6 %/h (–2.3 to –0.8 %/h). When compared with older models from the literature, performance was better with less overshoot after bolus doses.
Conclusion
The new pharmacokinetic model of hydromorphone showed a good precision and a better performance than older models. It is therefore suitable for TCI with hydromorphone during postoperative pain therapy.
Trial Registration
EudraCT 2013-002875-16, Clinical Trials NCT02035709.







Similar content being viewed by others
References
Struys MM, De Smet T, Mortier EP. Simulated drug administration: an emerging tool for teaching clinical pharmacology during anesthesiology training. Clin Pharmacol Ther. 2008;84:170–4.
Struys MM, Sahinovic M, Lichtenbelt BJ, Vereecke HE, Absalom AR. Optimizing intravenous drug administration by applying pharmacokinetic/pharmacodynamic concepts. Br J Anaesth. 2011;107:38–47.
van den Nieuwenhuyzen MC, Engbers FH, Vuyk J, Burm AG. Target-controlled infusion systems: role in anaesthesia and analgesia. Clin Pharmacokinet. 2000;38:181–90.
Palmer PP, Miller RD. Current and developing methods of patient-controlled analgesia. Anesthesiol Clinics. 2010;28:587–99.
Sidebotham D, Dijkhuizen MR, Schug SA. The safety and utilization of patient-controlled analgesia. J Pain Symptom Manage. 1997;14:202–9.
Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–23.
Dolin SJ, Cashman JN. Tolerability of acute postoperative pain management: nausea, vomiting, sedation, pruritus, and urinary retention. Evidence from published data. Br J Anaesth. 2005;95:584–91.
Jeleazcov C, Saari TI, Ihmsen H, Mell J, Frohlich K, Krajinovic L, et al. Population pharmacokinetic modeling of hydromorphone in cardiac surgery patients during postoperative pain therapy. Anesthesiology. 2014;120:378–91.
Westerling D, Björk H, Svedman P, Höglund P. Analgesic and nonanalgesic effects of intravenous hydromorphone—relation to plasma concentrations in healthy volunteers. Pain Res Manage. 1996;1:86–92.
Bonate PL. Pharmacokinetic-pharmacodynamic modeling and simulation. New York: Springer; 2006. p. 251–6.
Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88:1170–82.
Struys MM, Coppens MJ, De Neve N, Mortier EP, Doufas AG, Van Bocxlaer JF, et al. Influence of administration rate on propofol plasma-effect site equilibration. Anesthesiology. 2007;107:386–96.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
Saari TI, Fechner J, Ihmsen H, Schuttler J, Jeleazcov C. Analysis of total and unbound hydromorphone in human plasma by ultrafiltration and LC-MS/MS: application to clinical trial in patients undergoing open heart surgery. J Pharm Biomed Anal. 2012;71:63–70.
Varvel JR, Donoho DL, Shafer SL. Measuring the predictive performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm. 1992;20:63–94.
Swinhoe CF, Peacock JE, Glen JB, Reilly CS. Evaluation of the predictive performance of a ‘Diprifusor’ TCI system. Anaesthesia. 1998;53(Suppl 1):61–7.
Hill HF, Coda BA, Tanaka A, Schaffer R. Multiple-dose evaluation of intravenous hydromorphone pharmacokinetics in normal human subjects. Anesth Analg. 1991;72:330–6.
Parab PV, Ritschel WA, Coyle DE, Gregg RV, Denson DD. Pharmacokinetics of hydromorphone after intravenous, peroral and rectal administration to human subjects. Biopharm Drug Dispos. 1988;9:187–99.
The R Project for Statistical Computing. 2015. http://www.R-project.org/. Accessed 15 Oct 2015.
Rstudio. https://www.rstudio.com/. Accessed 15 Oct 2015.
Sherwin CM, Kiang TK, Spigarelli MG, Ensom MH. Fundamentals of population pharmacokinetic modelling: validation methods. Clin Pharmacokinet. 2012;51:573–90.
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, et al. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007;46:221–34.
Schuttler J, Kloos S, Schwilden H, Stoeckel H. Total intravenous anaesthesia with propofol and alfentanil by computer-assisted infusion. Anaesthesia. 1988;43(Suppl):2–7.
Glass PS, Shafer SL, Reves JG. Intravenous drug delivery systems. In: Miller RD, editor. Anesthesia. 5th ed. Philadelphia: Churchill Livingstone; 1999. p. 377–411.
Henthorn TK, Avram MJ, Krejcie TC. Intravascular mixing and drug distribution: the concurrent disposition of thiopental and indocyanine green. Clin Pharmacol Ther. 1989;45:56–65.
Acknowledgments
The authors thank Rainer Knoll, Dipl. Bioingenieur (Department of Anesthesiology, University of Erlangen-Nürnberg, Erlangen, Germany) for conducting the drug analysis. Else Huprich, M.A. (Department of Anesthesiology, University of Erlangen-Nürnberg, Erlangen, Germany), and Tobias Fuchte, Michael Kim, Sven Kremer and Alexander Weiß, Medical Students (Department of Anesthesiology, University of Erlangen-Nürnberg, Erlangen, Germany) are thanked for their assistance in data collection. Gabriele Göhring-Waldeck, Laboratory Technician (Department of Anesthesiology, University of Erlangen-Nürnberg, Erlangen, Germany) for her invaluable help in patient recruitment and study organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work has been supported by a grant of the German Federal Ministry of Education and Research, Berlin, Germany (Bundesministerium für Bildung und Forschung, Grant no: FKZ 13EX1015B).
Conflict of interest
Harald Ihmsen, Doris Rohde, Jürgen Schüttler and Christian Jeleazcov declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ihmsen, H., Rohde, D., Schüttler, J. et al. External Validation of a Recently Developed Population Pharmacokinetic Model for Hydromorphone During Postoperative Pain Therapy. Eur J Drug Metab Pharmacokinet 42, 17–28 (2017). https://doi.org/10.1007/s13318-015-0318-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-015-0318-x