Abstract
Background and Objective
Acyclovir is effective in treating herpes simplex virus infections of the central nervous system. The purpose of this study was to investigate the interactions between acyclovir and the efflux pumps P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp), multidrug resistance protein 2 (Mrp2), and organic anion transporter 3 (Oat3) at the blood–brain barrier (BBB).
Methods
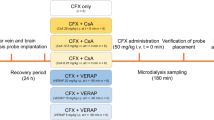
Acyclovir concentrations in the blood and brain were evaluated by microdialysis and high-performance liquid chromatography. Acyclovir pharmacokinetic parameters, including the area under the unbound blood concentration–time curve (AUCu,blood), the area under the unbound brain concentration–time curve (AUCu,brain), and the ratio of AUCu,brain to AUCu,blood (Kp.uu.brain), were evaluated in the presence and absence of elacridar (P-gp/Bcrp inhibitor, 7.5 mg/kg), tariquidar (P-gp/Bcrp inhibitor, 7.5 mg/kg), MK571 (Mrp2 inhibitor, 7.5 mg/kg), cyclosporine (P-gp/Bcrp/Mrp2 inhibitor, 25 mg/kg), and probenecid (Oat3 inhibitor, 50 mg/kg).
Results
The average AUCu,blood, AUCu,brain, and Kp.uu.brain in rats who received acyclovir (25 mg/kg, intravenous) alone were 1377.7 min · μg/ml, 435.4 min · μg/ml, and 31.6%, respectively. Probenecid drastically increased the AUCu,blood of acyclovir 1.73-fold, whereas coadministration with elacridar, tariquidar, MK571, and cyclosporine did not alter the blood pharmacokinetic parameters of acyclovir. Elacridar, tariquidar, MK571, cyclosporine, and probenecid significantly increased the AUCu,brain of acyclovir 1.51-, 1.54-, 1.47-, 1.95-, and 2.34-fold, respectively. Additionally, the Kp.uu.brain of acyclovir markedly increased 1.48-, 1.63-, 1.39-, 1.90-, and 1.35-fold following elacridar, tariquidar, MK571, cyclosporine, and probenecid administration, respectively.
Conclusion
The present study demonstrated that P-gp, Bcrp, Mrp2, and Oat3 inhibition increased the penetration of acyclovir across the BBB, supporting the hypothesis that these efflux pumps restrict the distribution of acyclovir in the brain.






Similar content being viewed by others
References
Furtado D, Bjornmalm M, Ayton S, Bush AI, Kempe K, Caruso F. Overcoming the blood-brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater. 2018;30(46):e1801362.
Konig J, Muller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65(3):944–66.
Shen S, Zhang W. ABC transporters and drug efflux at the blood-brain barrier. Rev Neurosci. 2010;21(1):29–53.
Mahringer A, Fricker G. ABC transporters at the blood-brain barrier. Expert Opin Drug Metab Toxicol. 2016;12(5):499–508.
Farthing CA, Sweet DH. Expression and function of organic cation and anion transporters (SLC22 family) in the CNS. Curr Pharm Des. 2014;20(10):1472–86.
Karbownik A, Stanislawiak-Rudowicz J, Stachowiak A, Romanski M, Grzeskowiak E, Szalek E. The influence of paracetamol on the penetration of sorafenib and sorafenib N-oxide through the blood-brain barrier in rats. Eur J Drug Metab Pharmacokinet. 2020;45(6):801–8.
Kato Y, Takahara S, Kato S, et al. Involvement of multidrug resistance-associated protein 2 (Abcc2) in molecular weight-dependent biliary excretion of beta-lactam antibiotics. Drug Metab Dispos. 2008;36(6):1088–96.
O’Brien FE, Clarke G, Fitzgerald P, Dinan TG, Griffin BT, Cryan JF. Inhibition of P-glycoprotein enhances transport of imipramine across the blood-brain barrier: microdialysis studies in conscious freely moving rats. Br J Pharmacol. 2012;166(4):1333–43.
Yanxiao C, Ruijuan X, Jin Y, et al. Organic anion and cation transporters are possibly involved in renal excretion of entecavir in rats. Life Sci. 2011;89(1–2):1–6.
Bharucha T, Houlihan CF, Breuer J. Herpesvirus infections of the central nervous system. Semin Neurol. 2019;39(3):369–82.
Wang W, Ji M. Efficacy of acyclovir for herpes simplex encephalitis: a protocol for a systematic review of randomized controlled trial. Medicine (Baltimore). 2019;98(15):e15254.
Lindstrom J, Hellden A, Lycke J, Grahn A, Studahl M. An unexpectedly high occurrence of aciclovir-induced neuropsychiatric symptoms in patients treated for herpesvirus CNS infection: a prospective observational study. J Antimicrob Chemother. 2019;74(12):3565–72.
Asahi T, Tsutsui M, Wakasugi M, et al. Valacyclovir neurotoxicity: clinical experience and review of the literature. Eur J Neurol. 2009;16(4):457–60.
Gunness P, Aleksa K, Koren G. Acyclovir is a substrate for the human breast cancer resistance protein (BCRP/ABCG2): implications for renal tubular transport and acyclovir-induced nephrotoxicity. Can J Physiol Pharmacol. 2011;89(9):675–80.
Liao XY, Deng QQ, Han L, et al. Leflunomide increased the renal exposure of acyclovir by inhibiting OAT1/3 and MRP2. Acta Pharmacol Sin. 2020;41(1):129–37.
Baltes S, Fedrowitz M, Tortos CL, Potschka H, Loscher W. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J Pharmacol Exp Ther. 2007;320(1):331–43.
Tollner K, Brandt C, Romermann K, Loscher W. The organic anion transport inhibitor probenecid increases brain concentrations of the NKCC1 inhibitor bumetanide. Eur J Pharmacol. 2015;5(746):167–73.
Mercolini L, Mandrioli R, Iannello C, Matrisciano F, Nicoletti F, Raggi MA. Simultaneous analysis of diazepam and its metabolites in rat plasma and brain tissue by HPLC-UV and SPE. Talanta. 2009;80(1):279–85.
Glowka FK, Hermann TW, Danielak D, Zabel M, Hermann J. Bioavailability of moclobemide from two formulation tablets in healthy humans. Pharmazie. 2019;74(2):97–100.
Liu H, Dong K, Zhang W, Summerfield SG, Terstappen GC. Prediction of brain:blood unbound concentration ratios in CNS drug discovery employing in silico and in vitro model systems. Drug Discov Today. 2018;23(7):1357–72.
Stahle L, Oberg B. Pharmacokinetics and distribution over the blood brain barrier of two acyclic guanosine analogs in rats, studied by microdialysis. Antimicrob Agents Chemother. 1992;36(2):339–42.
Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20(5):793–807.
Sane R, Agarwal S, Mittapalli RK, Elmquist WF. Saturable active efflux by P-glycoprotein and breast cancer resistance protein at the blood-brain barrier leads to nonlinear distribution of elacridar to the central nervous system. J Pharmacol Exp Ther. 2013;345(1):111–24.
Wang T, Agarwal S, Elmquist WF. Brain distribution of cediranib is limited by active efflux at the blood-brain barrier. J Pharmacol Exp Ther. 2012;341(2):386–95.
Kannan P, Telu S, Shukla S, et al. The “specific” P-glycoprotein inhibitor tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2). ACS Chem Neurosci. 2011;2(2):82–9.
Dickens D, Owen A, Alfirevic A, Pirmohamed M. ABCB1 single nucleotide polymorphisms (1236C>T, 2677G>T, and 3435C>T) do not affect transport activity of human P-glycoprotein. Pharmacogenet Genom. 2013;23(6):314–23.
Kuntner C, Bankstahl JP, Bankstahl M, et al. Dose-response assessment of tariquidar and elacridar and regional quantification of P-glycoprotein inhibition at the rat blood-brain barrier using (R)-[11C]verapamil PET. Eur J Nucl Med Mol Imaging. 2010;37(5):942–53.
Matzneller P, Kussmann M, Eberl S, et al. Pharmacokinetics of the P-gp inhibitor tariquidar in rats after intravenous, oral, and intraperitoneal administration. Eur J Drug Metab Pharmacokinet. 2018;43(5):599–606.
Neuwelt EA, Barnett P, Barranger J, McCormick C, Pagel M, Frenkel E. Inability of dimethyl sulfoxide and 5-fluorouracil to open the blood-brain barrier. Neurosurgery. 1983;12(1):29–34.
Lagas JS, van Waterschoot RA, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther. 2010;9(2):319–26.
Watanabe T, Kusuhara H, Maeda K, Shitara Y, Sugiyama Y. Physiologically based pharmacokinetic modeling to predict transporter-mediated clearance and distribution of pravastatin in humans. J Pharmacol Exp Ther. 2009;328(2):652–62.
Kusuhara H, Sugiyama Y. In vitro-in vivo extrapolation of transporter-mediated clearance in the liver and kidney. Drug Metab Pharmacokinet. 2009;24(1):37–52.
Worzfeld T, Schwaninger M. Apicobasal polarity of brain endothelial cells. J Cereb Blood Flow Metab. 2016;36(2):340–62.
Myint K, Li Y, Paxton J, McKeage M. Multidrug resistance-associated protein 2 (MRP2) mediated transport of oxaliplatin-derived platinum in membrane vesicles. PLoS ONE. 2015;10(7):e0130727.
Hasegawa Y, Kishimoto S, Shibatani N, et al. The pharmacokinetics of morphine and its glucuronide conjugate in a rat model of streptozotocin-induced diabetes and the expression of MRP2, MRP3 and UGT2B1 in the liver. J Pharm Pharmacol. 2010;62(3):310–4.
Takeuchi K, Shibata M, Kashiyama E, Umehara K. Expression levels of multidrug resistance-associated protein 4 (MRP4) in human leukemia and lymphoma cell lines, and the inhibitory effects of the MRP-specific inhibitor MK-571 on methotrexate distribution in rats. Exp Ther Med. 2012;4(3):524–32.
O’Brien FE, O’Connor RM, Clarke G, et al. The P-glycoprotein inhibitor cyclosporin A differentially influences behavioural and neurochemical responses to the antidepressant escitalopram. Behav Brain Res. 2014;15(261):17–25.
Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Curr Med Chem. 2012;19(13):1946–2025.
Li L, Yao QQ, Xu SY, et al. Cyclosporin A affects the bioavailability of ginkgolic acids via inhibition of P-gp and BCRP. Eur J Pharm Biopharm. 2014;88(3):759–67.
Marie S, Hernandez-Lozano I, Breuil L, et al. Validation of pharmacological protocols for targeted inhibition of canalicular MRP2 activity in hepatocytes using [99mTc]mebrofenin imaging in rats. Pharmaceutics. 2020;12(6):486.
Roberts LM, Black DS, Raman C, et al. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155(2):423–38.
Ose A, Ito M, Kusuhara H, et al. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos. 2009;37(2):315–21.
Li L, Agarwal S, Elmquist WF. Brain efflux index to investigate the influence of active efflux on brain distribution of pemetrexed and methotrexate. Drug Metab Dispos. 2013;41(3):659–67.
Horikawa M, Kato Y, Tyson CA, Sugiyama Y. The potential for an interaction between MRP2 (ABCC2) and various therapeutic agents: probenecid as a candidate inhibitor of the biliary excretion of irinotecan metabolites. Drug Metab Pharmacokinet. 2002;17(1):23–33.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Author contributions
YS, ZN, and JZ were involved in the conception and design; YS, YC, YZ, RT, and JZ performed the experiments and analyzed the samples; YS drafted the manuscript; ZN, JZ, and SY revised the paper. All the authors read and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work. All authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by the Military Medical Technology Youth Program of China [grant number 17QNP060].
Conflicts of interest
The authors declared that they have no conflicts of interest in relation to this work.
Ethics approval
All institutional and national guidelines for the care of the laboratory animals were followed. All experimental protocols involving animals were reviewed and approved by the Committee on Animal Use for Research and Education of the Laboratory Animals Centre, General Hospital of Chinese People’s Liberation Army (Beijing, China).
Data availability statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
About this article
Cite this article
Shan, Y., Cen, Y., Zhang, Y. et al. Acyclovir Brain Disposition: Interactions with P-gp, Bcrp, Mrp2, and Oat3 at the Blood–Brain Barrier. Eur J Drug Metab Pharmacokinet 47, 279–289 (2022). https://doi.org/10.1007/s13318-021-00733-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-021-00733-w



