Abstract
Introduction
To evaluate the safety and effectiveness of insulin glargine 300 U/mL (Gla-300) in people with type 2 diabetes mellitus (T2DM) in the Gulf region who fast during Ramadan.
Methods
ORION was a real-world, prospective, observational study in people with T2DM treated with Gla-300 during pre-Ramadan, Ramadan, and post-Ramadan periods. This subgroup analysis included 222 participants from the Gulf region (Kuwait, Saudi Arabia, United Arab Emirates, and Qatar). The primary endpoint was the percentage of participants experiencing severe and/or symptomatic documented hypoglycemia (self-monitored plasma glucose [SMPG] ≤ 70 mg/dL) during Ramadan. Changes in glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), SMPG, body weight, insulin dose, and adverse events (AEs) were also evaluated.
Results
The primary endpoint was reported in one (0.5%) participant during Ramadan. The incidence rate of symptomatic documented hypoglycemia (SMPG ≤ 70 mg/dL) decreased from the pre-Ramadan (3.2%) to Ramadan period (0.5%), and no severe hypoglycemia events were reported during the study. Reductions were observed in HbA1c (mean ± standard deviation: − 0.51 ± 0.95% [− 5.5 ± 10.4 mmol/mol]), FPG (− 13.9 ± 47.5 mg/dL), and SMPG (− 6.1 ± 27.1 mg/dL). No significant changes were observed in body weight or Gla-300 dose. AEs were reported in 11 (5.0%) participants.
Conclusion
In a real-world setting in the Gulf region, Gla-300 treatment in people with T2DM during Ramadan was associated with a low incidence of hypoglycemia and improved glycemic control.
Trial Registration
CTRI/2019/02/017636.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Despite the challenges associated with diabetes and fasting during Ramadan, most Muslim individuals with diabetes observe the Ramadan fast due to their religious and personal beliefs and motivation. |
The International Diabetes Federation and the Diabetes and Ramadan International Alliance Practical Guidelines recommend the use of basal insulin analogs during Ramadan due to the lower risk of hypoglycemia compared to regular human insulin; however, there is a paucity of studies evaluating the efficacy and safety of the second-generation basal insulin analog use during Ramadan. |
The ORION study (real-world, prospective, observational study across 11 countries) reported that the use of insulin glargine 300 U/mL (Gla-300) was associated with favorable outcomes in people with type 2 diabetes mellitus (T2DM) who fasted during the Ramadan period. |
This subgroup analysis evaluated the safety and effectiveness of Gla-300 in the T2DM subpopulation from the Gulf region. |
What was learned from this study? |
In the present analysis, participants with T2DM in the Gulf region who were treated with Gla-300 during Ramadan had a few episodes of hypoglycemia during the Ramadan period, with the rate comparable with that of the overall population, and there was no occurrence of severe hypoglycemia during the study period. |
Further, participants had improved glycemic control from the pre-Ramadan to the post-Ramadan period, similar to that observed in the overall population. |
These results support the safety and effectiveness of Gla-300 in people with T2DM who fast during Ramadan and demonstrate that Gla-300 offers an effective treatment option with a low risk of hypoglycemia for those requiring insulin therapy. |
Introduction
The age-adjusted prevalence of diabetes is estimated to be the highest in the Middle East and North Africa (MENA) region. According to the latest International Diabetes Federation (IDF) figures, 73 million people (aged 20–79 years) were living with diabetes in the MENA region in 2021, and this number is estimated to increase to 136 million by 2045 [1]. Glycemic control remains suboptimal in the MENA region [2]. Insulin is recommended for people with type 2 diabetes mellitus (T2DM) whose disease is inadequately controlled despite treatment with optimal oral antidiabetic drug (OAD) therapy [3,4,5]. However, initiation of insulin therapy is often delayed in people with T2DM [2] due to the fear of hypoglycemia and its associated morbidity [6].
Fasting during Ramadan further increases the risk of hypoglycemia [7, 8], with a particularly high incidence reported in the Gulf region [9]. Previous studies have reported a 4.5- to 7.5-fold increased risk of hypoglycemia in people with T2DM who fasted during Ramadan [9, 10]. Also, recent studies have shown that the risk of hypoglycemia during Ramadan is higher in insulin-treated people with T2DM than in those receiving OADs [9, 10]. Additionally, the use of insulin in people receiving multiple antidiabetic agents is also associated with higher rates of hypoglycemia than in those who are not on insulin [11]. Basal insulin analogs reduce the risk of hypoglycemia, especially of nocturnal hypoglycemia, compared with regular human insulin. Therefore, the IDF and Diabetes and Ramadan (DAR) International Alliance (IDF-DAR) Practical Guidelines recommend the use of basal insulin analogs over regular human insulin for people who fast during Ramadan [12].
Insulin glargine 300 U/mL (Gla-300) is a second-generation basal insulin analog that has demonstrated comparable glycemic control but with a lower incidence of hypoglycemia than the first-generation analog, insulin glargine 100 U/mL, in adults with T2DM [13,14,15]. However, there is paucity of data evaluating the safety and effectiveness of Gla-300 in people with T2DM during Ramadan fasting in the Gulf region.
The real-world ORION study conducted in 11 countries in the MENA, South-East Asia, and Canada showed that people with T2DM receiving Gla-300 who fasted during Ramadan had a low risk of severe/symptomatic hypoglycemia and experienced an improvement in glycemic control [16]. We have undertaken a subgroup analysis of the ORION study to evaluate the safety and effectiveness of Gla-300 during the pre-Ramadan, Ramadan, and post-Ramadan periods in participants with T2DM specifically from the Gulf region.
Methods
Study Design
The design and methods of the ORION study have been previously described [16]. Briefly, the ORION study (World Health Organization International Clinical Trials Registry Platform: CTRI/2019/02/017636) was a prospective, observational, international multicenter study. A total of 493 adult participants with T2DM were enrolled in the study, with the aim to evaluate the safety and effectiveness of Gla-300 during the pre-Ramadan (1–3 months prior to Ramadan), Ramadan (1 month), and post-Ramadan (1 month) periods. Data were collected at three study visits: one each during the selection/recruitment (3 months prior to Ramadan), pre-Ramadan, and post-Ramadan periods, respectively. Data on diabetes complications and medical history were collected during the pre-Ramadan visit and recorded in the electronic case record form per the physician’s assessment. Participants kept diaries on glucose values, basal insulin doses, episodes of symptomatic hypoglycemia, and days with/without fasting. Participants who discontinued Gla-300 treatment during the study were observed until the end of the study. Written informed consent was obtained from each participant. This study was conducted in accordance with the Declaration of Helsinki of 1964 and all subsequent amendments. The study protocol was approved by the local institutional review board/independent ethics committee of the country of each participating investigator, and regulatory submissions were performed in accordance with the local data protection guidelines. The present analysis included participants from four countries in the Gulf region: Kuwait, Saudi Arabia, United Arab Emirates, and Qatar.
Inclusion and Exclusion Criteria
All adults (aged ≥ 18 years or at a legal age of adulthood) with T2DM who were receiving basal insulin therapy with Gla-300, prescribed per routine clinical practice, for ≥ 8 weeks and planned to continue Gla-300 treatment and fast for ≥ 15 days during Ramadan were eligible for enrollment in the study. Insulin dose adjustments were made based on the advice of the treating physician. Participants who used basal-bolus or premix insulin within 6 months or any investigational drug within 1 month or 5 half-lives prior to the selection visit and had a known hypersensitivity/intolerance to Gla-300 (or its excipients) and women who were pregnant or breastfeeding were excluded.
Endpoints
The primary objective was to assess the percentage of participants experiencing ≥ 1 severe and/or symptomatic documented hypoglycemia event with self-monitored plasma glucose (SMPG) ≤ 70 mg/dL in all the three study periods, with the primary endpoint analyzed during the Ramadan period. Secondary endpoints included incidence of ≥ 1 severe and/or symptomatic hypoglycemia event with SMPG < 54 mg/dL and event rates of severe and/or symptomatic hypoglycemia with SMPG ≤ 70 mg/dL and < 54 mg/dL, assessed at three predefined time periods and by time of day (daytime [06:00 am to 11:59 pm] and nocturnal [12:00 am to 05:59 am] in the pre- and post-Ramadan periods, and fasting [between Suhur and Iftar] and non-fasting [between Iftar and Suhur] periods during Ramadan). Hypoglycemia events were assessed per the American Diabetes Association classification, defined as level 1 if blood glucose < 70 mg/dL (3.9 mmol/L), level 2 if blood glucose < 54 mg/dL (3.0 mmol/L) and needs immediate action, and level 3 as a severe event characterized by altered mental and/or physical status requiring assistance [17]. Other secondary endpoints included mean changes in glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and SMPG (assessed during pre- and post-Ramadan periods); mean fasting SMPG before Iftar (evening meal) during Ramadan; change in Gla-300 dose from the pre-Ramadan to the Ramadan and to the post-Ramadan periods; and mean change in body weight from the pre-Ramadan to the post-Ramadan period. Adverse events (AEs), including episodes of hyperglycemia, and serious AEs were also assessed.
Analyses
Both primary and secondary endpoints were evaluated in eligible participants from the Gulf region. The primary endpoint was analyzed during the Ramadan period and reported as proportion of participants experiencing ≥ 1 episode of severe and/or symptomatic documented hypoglycemia with SMPG ≤ 70 mg/dL, with two-sided 95% confidence interval (CI), adjusted for study duration and participants lost to follow-up. The secondary endpoint (number of participants experiencing ≥ 1 event of severe and/or symptomatic documented hypoglycemia with SMPG < 54 mg/dL across the three periods) was analyzed per the primary endpoint analysis. The event rates of severe and/or symptomatic hypoglycemia (SMPG ≤ 70 mg/dL and < 54 mg/dL, respectively) during the pre-Ramadan, Ramadan, and post-Ramadan periods were assessed across 1 to 3 months for the pre-Ramadan period, across 1 month for the Ramadan period, and across 1 to 2 months for the post-Ramadan period. The secondary endpoints of effectiveness, safety, change in Gla-300 dose, and body weight were summarized using descriptive statistics.
Results
This ORION subgroup analysis included 222 participants with T2DM recruited at 27 centers in the Gulf region (Kuwait, n = 86; Saudi Arabia, n = 51; United Arab Emirates, n = 50; and Qatar, n = 35). Most participants completed the study (205 [92.3%]) and continued to be treated with Gla-300 until the end of the study (220 [99.1%] participants at final evaluation). Only two participants permanently discontinued Gla-300; the reason for discontinuation was an AE in one participant and cost of the treatment in the second participant.
Baseline Demographics and Participant Characteristics
At baseline, the mean ± standard deviation (SD) age was 55.6 ± 11.3 years, and 116 (52.3%) of the participants were male (Table 1). The mean (± SD) body mass index (BMI) was 30.1 ± 5.2 kg/m2, and 81.0% of the participants had a BMI of 25–40 kg/m2. The mean (± SD) duration of diabetes was 12.8 ± 7.9 years, and 128 (57.7%) participants had a diabetes duration of ≥ 10 years. The median time since the first insulin treatment was 1.3 (quartile 1: quartile 3 [Q1:Q3], 0.4:3.9) years, and the median duration of Gla-300 treatment was 5.6 (Q1:Q3, 3.0:12.0) months.
Before study initiation, the recommended frequency of Gla-300 dose adjustments was at least weekly for the majority (159, 78.7%) of participants. An increment of two units per dose adjustment step was recommended for 167 (82.7%) of the participants. The mean (± SD) HbA1c target was 6.9 ± 0.4% (52.0 ± 4.3 mmol/mol), and 21 (11.2%) participants were at their HbA1c target during the pre-Ramadan period. When fasting, a major proportion (172, 78.5%) of participants were at a moderate/low risk of complications, as determined by their physician, while 34 (15.5%) and 13 (5.9%) of participants were at high and very high risk, respectively. Three quarters (167, 75.2%) of the participants were treated with ≥ 2 non-insulin anti-hyperglycemic treatments, the most common being biguanides (77.0%), followed by dipeptidyl peptidase-4 (DPP-4) inhibitors (42.8%), sulfonylureas (SU; 40.5%), and sodium glucose cotransporter-2 inhibitors (29.3%). The proportion of participants taking various classes of non-insulin anti-hyperglycemic treatments and the proportion of participants treated with ≥ 2 non-insulin anti-hyperglycemic treatments were similar at study initiation and the pre-Ramadan period (Table 2). Further, very few changes were observed in background therapy during the Ramadan period.
Nearly half of the participants (106, 47.7%) reported having a history of any diabetes-related complication or comorbidity (Electronic Supplementary Material [ESM] Table S1). Diabetic neuropathy was the most commonly reported comorbidity (36.5%), followed by impaired renal function (23.0%) and diabetic retinopathy (20.3%). Twenty-three (10.4%) participants were reported to have at least one macrovascular complication. Complications and comorbidities, including myocardial infarction, heart failure, atrial fibrillation, stroke, transient ischemic attack, peripheral vascular disease, and history of hypoglycemia unawareness, were reported in a small fraction (2–4%) of participants.
A total of 188 (89.1%) participants observed the fast for the entire Ramadan period (mean [± SD] duration of fasting: 30.5 ± 2.5 days). Twenty-three (10.9%) participants broke their fast at least once; the reasons reported for breaking the fast were hypoglycemia (n = 1), travel (n = 2), menses (n = 5), illness (n = 11), and others (n = 2); data were missing for two participants. For 187 (88.6%) participants, Gla-300 injection during Ramadan was recommended in the evening (at Iftar), and all of these participants complied with this recommendation. The majority (198, 93.4%) of participants reported complete adherence to Gla-300 daily treatment.
Severe and/or Symptomatic Documented Hypoglycemia
Overall, the number (%) of participants experiencing ≥ 1 event of severe and/or symptomatic documented hypoglycemia with SMPG ≤ 70 mg/dL during the study was low in the pre-Ramadan period (7 [3.2%], 95% CI 1.3–6.4) and further decreased during the Ramadan (1 [0.5%], 95% CI 0.0–2.5) and post-Ramadan periods (1 [0.5%], 95% CI 0.0–2.5) (Fig. 1). Only one participant experienced ≥ 1 event of symptomatic documented hypoglycemia with SMPG < 54 mg/dL, which occurred in the pre-Ramadan period. No symptomatic documented hypoglycemia with SMPG < 54 mg/dL was reported during the Ramadan or post-Ramadan periods (ESM Table S2). No severe hypoglycemia events were reported during the study. The event rate for severe and/or symptomatic documented hypoglycemia with SMPG ≤ 70 mg/dL was 0.018 per participant-month of follow-up (PPM) in the pre-Ramadan, 0.005 PPM during the Ramadan, and 0.006 PPM in the post-Ramadan period.
The number of participants experiencing daytime versus nocturnal symptomatic hypoglycemia (SMPG ≤ 70 mg/dL) was 4 (1.8%) versus 3 (1.4%) in pre-Ramadan period and one (0.5%) versus none in the post-Ramadan period, respectively, and all hypoglycemia events occurred between Suhur and Iftar in the participant who experienced hypoglycemia during Ramadan.
Changes in Glycemic Endpoints (HbA1c, FPG, and SMPG)
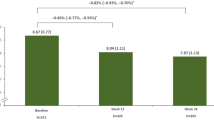
A reduction in HbA1c was observed from the pre-Ramadan (8.27 ± 1.35% [66.9 ± 14.7 mmol/mol]) to the post-Ramadan (7.73 ± 1.11% [61.0 ± 12.2 mmol/mol]) period (Fig. 2a). A mean (± SD) reduction of 13.9 ± 47.5 mg/dL and 6.1 ± 27.1 mg/dL was observed in FPG and SMPG, respectively, from the pre-Ramadan to the post-Ramadan period (Fig. 2b, c).
Change in Insulin Dose
The mean Gla-300 daily dose was 0.350 ± 0.147 U/kg during the pre-Ramadan period, which decreased to 0.343 ± 0.141 U/kg during the Ramadan period (mean difference: − 0.007 ± 0.083 U/kg) and increased to 0.359 ± 0.147 U/kg in the post-Ramadan period. The mean change in the Gla-300 dose from the pre-Ramadan to the post-Ramadan period was 0.004 ± 0.081 U/kg (Fig. 2d).
Change in Body Weight
There was no significant change in body weight from the pre-Ramadan (83.3 ± 15.4 kg) to the post-Ramadan period (83.0 ± 15.4 kg), with a mean (± SD) difference of − 0.5 ± 2.5 kg.
Adverse Events
Incidences of AEs were low throughout the study period (Table 3). A total of 11 (5.0%) participants experienced at least one AE throughout the study period, including nine (4.1%) participants who experienced the AE during Ramadan. Three (1.4%) participants had a serious AE, with one (0.5%) participant experiencing a serious AE during Ramadan. One participant discontinued the Gla-300 treatment during Ramadan due to ovarian adenoma. None of the AEs reported were considered to be related to Gla-300, and there were no deaths due to an AE during the study period.
Overall, one participant experienced at least one AE of hyperglycemia, which occurred during Ramadan and was neither considered serious nor related to Gla-300 and did not result in discontinuation of Gla-300.
Discussion
Only a limited number of studies have assessed the effect of basal insulin during Ramadan. The real-world, prospective, observational ORION study was undertaken to address this gap and provide more insight into the role of Gla-300 as a treatment option in people with T2DM who fast during Ramadan [16]. The ORION study showed that the incidence of severe and/or symptomatic hypoglycemia was low among the study population and that there was an improvement in glycemic parameters from the pre-Ramadan to the post-Ramadan periods, supporting the use of Gla-300 in people with T2DM who fast during Ramadan [16].
The present analysis of the ORION study included participants with T2DM from the Gulf region who were treated with Gla-300. The majority were able to fast for the entire Ramadan period. When compared with the overall population recruited into the ORION study, the subpopulation of participants in the Gulf region had a longer duration of diabetes (12.8 vs 10.7 years) and a greater proportion had comorbidities (47.7% vs. 38.9%), with a very high risk of hypoglycemia (5.9% vs. 2.9%), and the HbA1c target achievement was lower (11.2% vs. 15.3%).
The reported incidence and event rates of severe and/or symptomatic hypoglycemia were lower in the participants in the Gulf region than in the overall population. Notably, prior and concomitant DPP-4 inhibitor and SU use was lower in the participants in the Gulf region than in the overall population in the ORION study [16], which might have contributed to fewer hypoglycemia events in the former. Another important observation was the reduction in hypoglycemia events from the pre-Ramadan (3.2%) to the post-Ramadan period (0.5%), despite minimal reductions in the Gla-300 dose. Ba-Essa et al. conducted a prospective, non-randomized study in Saudi Arabia over two consecutive years, with the aim to evaluate safety among 360 people with T2DM who used various antidiabetic treatments and fasted during Ramadan [10]. These authors reported that the incidence of hypoglycemia during the Ramadan period was highest in people receiving insulin, either as monotherapy (46.9%) or in combination with OADs (35.2%). However, the risk of hypoglycemia was reported to be lower when basal insulin was used in combination with non-SU OADs [10]. Additionally, two real-world observational studies, DAR-MENA [7] and CREED [18], have evaluated diabetes management and outcomes in people with T2DM during Ramadan across multiple countries, including from the Middle East region. Results from the CREED study show that the incidence (4.1% vs. 13% and 6.7%) and risk of hypoglycemia were lower in the Middle East region than in North Africa and Europe, despite a higher use of insulin either alone or in combination with OADs (26.9% vs. 20.1% and 22%) and SU (74.3% vs. 64.2% and 36.8%) [18]. This may explain the lower hypoglycemia incidence observed in the participants in the ORION Gulf region than in overall population. Furthermore, similar to the present analysis, a decrease in hypoglycemia incidence from the pre-Ramadan to the Ramadan period was observed in the Middle East region in the CREED study, including in those receiving insulin [18]. In contrast to this result, a significant increase in confirmed hypoglycemia events was reported from the pre-Ramadan to the Ramadan period (incidence: 4.9–10.4%; rate: 0.11–0.22 events/month/participant) in the DAR-MENA study [7]. It is possible that this difference may be due to the higher risk of hypoglycemia in the North Africa region than in the Middle East, as observed in the CREED study [18]. Further studies are required to assess the impact of changes in OAD class and dosing on hypoglycemia events during Ramadan in people treated with Gla-300. Nevertheless, the present results are promising, considering that 75.2% of participants were treated with ≥ 2 concomitant non-insulin medications and 40.5% of participants were treated with an SU.
While the study population in the present analysis had more comorbidities than the overall population in the ORION study, no severe or symptomatic hypoglycemia with an SMPG < 54 mg/dL was reported during the Ramadan or the post-Ramadan period, which is consistent with the results of the overall population. Similarly, Ba-Essa et al. reported severe hypoglycemia during Ramadan fasting in 1% of the study participants [10], in line with the present findings.
In the Gulf population, symptomatic documented hypoglycemia at ≤ 70 mg/dL SMPG threshold was recorded in only one (0.5%) participant during the Ramadan period (occurred during the fasting hours) versus 13 (2.6%) participants in the overall ORION population. Of note, approximately one half of the participants reporting these events in the overall population were from Canada, where the fasting hours are longer (≥ 18 h) [16, 19]. In addition, a higher proportion of participants in Canada achieved their HbA1c target at baseline compared with the overall population, which might have increased the risk of hypoglycemia [16].
All parameters of glycemic control, including HbA1c, FPG, and fasting SMPG levels, improved from the pre-Ramadan to the post-Ramadan period in both the overall population and the Gulf population. These observations are in line with the observations reported in previously published Ramadan studies. The DAR-MENA study reported a significant improvement in HbA1c (− 0.5%) and FPG (− 16.4 mg/dL) levels following Ramadan (P < 0.0001) [7]. Ba-Essa et al. also reported a significant reduction in mean HbA1c (− 0.2%; P < 0.022) during Ramadan fasting [10]. There was a marginal increase in Gla-300 dose (mean ± SD: 0.004 ± 0.081 U/kg) from the pre-Ramadan to the post-Ramadan period in the Gulf population, which was similar to that seen in the overall population (0.005 ± 0.07 U/kg) [16]. Further, similar to the ORION overall population [16], only 19.0% of participants in the Gulf region reduced their Gla-300 dose by ≥ 15% (recommended by the IDF-DAR guidelines [12]), which could be attributed to: (1) the Gla-300 dose being already low in the pre-Ramadan period and (2) the majority of participants (88.8%) being not at their HbA1c target. Additionally, the trend observed for weight was similar for both the overall ORION and Gulf populations, with a mean difference of − 0.5 ± 2.3 kg and − 0.5 ± 2.5 kg, respectively [16]. A similar pattern of weight change was observed in the DAR-MENA study (− 0.6 ± 4.1 kg; P < 0.0001) from the pre-Ramadan to the post-Ramadan period; however, the change was not considered to be clinically significant [7]. On the other hand, Ba-Essa et al. reported no change in weight in most participants (70.9%) [10].
Overall, the present findings from the Gulf region subgroup analysis of the ORION study have shown that despite including participants with a longer duration of diabetes, more comorbidities, and higher risk of hypoglycemia, there was no excess occurrence of either mild or severe episodes of hypoglycemia during Ramadan. This finding further supports the safety and effectiveness of Gla-300 in people with T2DM who fast during Ramadan. Given that most people with T2DM in the Gulf region choose to fast during Ramadan, including those in high-risk categories [7, 10], Gla-300 offers an effective treatment option with a low risk of hypoglycemia for those requiring insulin therapy. The present study enriches the body of evidence supporting the safety of basal insulin in people with T2DM who choose to fast during Ramadan.
While this is not a limitation of the present subanalysis, it is worth noting that the study was an observation real-world study and, hence, had inherent disadvantages, including the risk of confounding and bias, and lack of randomization. Furthermore, participants in the ORION study could fast for ≥ 15 days and were at moderate/low risk of complications and therefore may not fully reflect the safety of Gla-300 in a high-/very high-risk population according to the IDF-DAR guidelines [12].
Conclusion
In the present subgroup analysis of the ORION study, people in the Gulf region with T2DM treated with Gla-300 during Ramadan had no severe hypoglycemia, a low risk of symptomatic hypoglycemia, and improved glycemic control. This was achieved even though the participants from the Gulf region had more comorbidities and a longer duration of diabetes, which are the two factors known to augment the risk of hypoglycemia. The results suggest that Gla-300 may be a suitable treatment option for people with T2DM who intend to fast during Ramadan.
References
International Diabetes Federation. IDF diabetes atlas, 10th ed. 2021. www.diabetesatlas.org. Accessed 19 Dec 2021.
Jabbar A, Abdallah K, Hassoun A, et al. Patterns and trends in insulin initiation and intensification among patients with type 2 diabetes mellitus in the Middle East and North Africa region. Diabetes Res Clin Pract. 2019;149:18–26.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020(43):487–93.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S111–24.
Khunti K, Alsifri S, Aronson R, et al. Impact of hypoglycaemia on patient-reported outcomes from a global, 24-country study of 27,585 people with type 1 and insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2017;130:121–9.
Hassanein M, Al Awadi FF, El Hadidy KES, et al. The characteristics and pattern of care for the type 2 diabetes mellitus population in the MENA region during Ramadan: an international prospective study (DAR-MENA T2DM). Diabetes Res Clin Pract. 2019;151:275–84.
Salti I, Benard E, Detournay B, et al. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. 2004;27:2306–11.
Beshyah SA, Hassanein M, Ahmedani MY, et al. Diabetic hypoglycaemia during Ramadan fasting: a trans-national observational real-world study. Diabetes Res Clin Pract. 2019;150:315–21.
Ba-Essa EM, Hassanein M, Abdulrhman S, et al. Attitude and safety of patients with diabetes observing the Ramadan fast. Diabetes Res Clin Pract. 2019;152:177–82.
Elhadd T, Dabbous Z, Bashir M, et al. Incidence of hypoglycaemia in patients with type-2 diabetes taking multiple glucose lowering therapies during Ramadan: the PROFAST Ramadan Study. J Diabetes Metab Disord. 2018;17:309–14.
International Diabetes Federation and the DAR International Alliance. Diabetes and Ramadan: practical guidelines. 2021. https://www.daralliance.org/daralliance/idf-dar-practical-guidelines-2021/. Accessed 02 Dec 2021.
Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naive people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386–94.
Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755–62.
Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235–43.
Hassanein M, Akif Buyukbese M, Malek R, et al. Real-world safety and effectiveness of insulin glargine 300 U/mL in participants with type 2 diabetes who fast during Ramadan: the observational ORION study. Diabetes Res Clin Pract. 2020;166:108189.
Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40:1622–30.
Jabbar A, Hassanein M, Beshyah SA, et al. CREED study: hypoglycaemia during Ramadan in individuals with Type 2 diabetes mellitus from three continents. Diabetes Res Clin Pract. 2017;132:19–26.
Bajaj HS, Abouhassan T, Ahsan MR, et al. Diabetes Canada position statement for people with types 1 and 2 diabetes who fast during Ramadan. Can J Diabetes. 2019;43:3–12.
Acknowledgements
The authors thank all participants, study investigators, and staff who participated in the data collection for the study (participating physicians from the Gulf region are listed in Appendix I of the ESM). The authors acknowledge Baptiste Berthou (Statistician of IT&M Stats fully contracted to Sanofi) and Valérie Pilorget (Senior Director Clinical Research of Sanofi) for their contribution to the conceptualization of the work, statistical analysis, and interpretation of the data. The authors also thank Maria Aileen Mabunay for her critical inputs during the manuscript development. Coordination for the development of this manuscript and assistance with the revision was provided by Sirisha Pedapudi of Sanofi.
Funding
The ORION study and the journal’s Rapid Service Fee were funded by Sanofi.
Medical Writing Assistance
Scientific writing and editorial support were provided by Jyothi Ramanathan and Preeti Agarwal, who are employees of Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author Contributions
Mohamed Hassanein and Mubarak Naqvi were involved in the conception and design of the study. Mohamed Hassanein, Rayaz A Malik, Tarik Elhadd, Abdulnabi Alattar, Abdulrahman Al Shaikh, Muneera Al Randi, Ravi Arora, Saud Al-Sifri, Yasser Akil, and Mubarak Naqvi contributed to data acquisition for the work related to this document. All authors substantially contributed to the data analysis/interpretation of the results, critically reviewed, and approved the final version for submission, and are accountable for the accuracy and integrity of this manuscript.
Prior Presentation
Data was partially presented at the American Diabetes Association—80th Scientific Session (virtual, 12–16 June 2020) and at the Diabetes & Ramadan International Alliance—8th Conference (Dubai, 24–25 January, 2020).
Disclosures
Abdulnabi Alattar and Abdulrahman Al Shaikh has no conflict of interests to disclose. Rayaz A Malik has attended advisory boards and received honorarium for lectures from Novo Nordisk, Sanofi, AstraZeneca, and Boehringer Ingelheim. Tarik Elhadd has served as consultant and speaker for Sanofi, Novo Nordisk, MSD, and Merck. Muneera Al Randi has served as advisor and speaker for Sanofi, Novo Nordisk, AstraZeneca, and Boehringer Ingelheim. Ravi Arora has received payments for lectures and continuing medical education events as speaker, and reimbursements for travel and accommodation expenses for national and international conferences. Saud Al-Sifri has served as adviser and speaker for AstraZeneca, Boehringer Ingelheim, Lilly, MSD, Novo Nordisk, Sanofi, and Novartis. Mohamed Hassanein has served as member of the advisory board of Sanofi, Boehringer Ingelheim, and Novo Nordisk; received a speaker honorarium from Eli Lilly, Janssen, LifeScan, Merck Sharp and Dohme, Novo Nordisk, and Sanofi; and has received lecture/other fees from Sanofi, Novo Nordisk, Eli Lilly, Merck Sharp and Dohme, Janssen, and LifeScan. Yasser Akil, Amr Magdy, and Mubarak Naqvi are employees of Sanofi and may hold Sanofi stock/shares.
Compliance with Ethics Guidelines
This study was conducted in accordance with the Declaration of Helsinki of 1964 and all subsequent amendments. The study protocol was approved by the local institutional review board/independent ethics committee of the country of each participating investigator, and regulatory submissions were performed in accordance with the local data protection guidelines. Written informed consent was obtained from each participant.
Data Availability
Qualified researchers may request access to patient-level data and related documents (including, for example, the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications). Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the Electronic Supplementary Material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Malik, R.A., Elhadd, T., Alattar, A. et al. Safety and Effectiveness of Insulin Glargine 300 U/mL in Participants with Type 2 Diabetes Who Fast During Ramadan in The Gulf Region: A Subgroup Analysis of the Real-World ORION Study. Diabetes Ther 13, 569–581 (2022). https://doi.org/10.1007/s13300-022-01225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01225-z