Abstract
Introduction
The clinical benefits of insulin glargine 300 U/mL (Gla-300) have been confirmed in randomised clinical trials (EDITION programme and BRIGHT) and real-world studies in the USA and Western Europe. ATOS evaluated the real-world effectiveness and safety of Gla-300 in wider geographic regions (Asia, the Middle East, North Africa, Latin America and Eastern Europe).
Methods
This prospective observational, international study enrolled adults (≥ 18 years) with type 2 diabetes mellitus (T2DM) uncontrolled [haemoglobin A1c (HbA1c) > 7% to ≤ 11%] on one or more oral anti-hyperglycaemic drugs (OADs) who had been advised by their treating physician to add Gla-300 to their existing treatment. The primary endpoint was achievement of a pre-defined individualised HbA1c target at month 6.
Results
Of the 4550 participants included, 4422 (51.8% female) were eligible for assessment. The mean ± standard deviation (SD) age was 57.2 ± 10.8 years, duration of diabetes was 10.2 ± 6.2 years and baseline HbA1c was 9.28 ± 1.0%. The proportion of participants reaching their individualised glycaemic target was 25.2% [95% confidence interval (CI) 23.8–26.6%] at month 6 and 44.5% (95% CI 42.9–46.1%) at month 12. At months 6 and 12, reductions were observed in HbA1c (−1.50% and −1.87%) and fasting plasma glucose (−3.42 and −3.94 mmol/L). Hypoglycaemia incidence was low, and body weight change was minimal. Adverse events were reported in 283 (6.4%) participants, with 57 (1.3%) experiencing serious adverse events.
Conclusion
In a real-world setting, initiation of Gla-300 in people with T2DM uncontrolled on OADs resulted in improved glycaemic control and low rates of hypoglycaemia with minimal weight change.
Trial Registration
Clinicaltrials.gov number NCT03703869.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Efficacy and safety of insulin glargine 300 U/mL (Gla-300) has been demonstrated in randomised clinical trials and real-world studies conducted in the USA and Western Europe. |
ATOS study evaluated the use of Gla-300 in real-world populations across Asia, the Middle East, North Africa, Latin America and Eastern Europe. |
What was learned from the study? |
Initiation of Gla-300 in insulin-naïve participants with type 2 diabetes after failure of oral anti-hyperglycaemic drugs, showed an improvement in glycaemic control with an adequate safety profile. |
On the basis of the current study results, in a real-world clinical setting, Gla-300 can be a treatment choice for people with type 2 diabetes who are insufficiently controlled with oral anti-hyperglycaemic drugs across the five regions of the world with diverse clinical profiles and treatment practices. |
Digital Features
This article is published with a graphical abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.21070690.
Introduction
Diabetes is a major health issue that affects 463 million adults worldwide, the majority (90%) of whom have type 2 diabetes mellitus (T2DM). Furthermore, its prevalence is rapidly rising, especially in Africa, the Middle East, South East Asia and Central America regions [1]. In developing countries, the major factors driving the increased prevalence are unhealthy lifestyle factors, such as diet and sedentary lifestyle [2], genetic susceptibility [3, 4] and unique patient phenotypes such as higher glycated haemoglobin (HbA1c) levels, and low degree of initial presentation of obesity, especially in Asia [5,6,7,8]. Management of T2DM in these settings is suboptimal, often owing to challenges with access to medications and increased co-morbid disorders associated with diabetes.
Insulin glargine 300 U/mL (Gla-300) is a second-generation basal insulin analogue, which has a more stable and prolonged pharmacokinetic and pharmacodynamic profile compared with the first‐generation basal insulin analogue, insulin glargine 100 U/mL (Gla‐100) [9]. The EDITION randomised controlled trial (RCT) programme demonstrated that Gla-300 provided similar glycaemic control with lower hypoglycaemia risk compared with Gla‐100 in adults with T2DM [10,11,12,13,14]. Nevertheless, results obtained in RCTs are not always representative of real-world scenarios; thus, observational studies in a real-world setting are important to validate the results from RCTs. The clinical benefits of Gla-300 observed in RCTs have subsequently been confirmed in real-world studies conducted in the USA and Western Europe [15,16,17,18]; however, given that 75% of people with diabetes reside outside these regions, real-world data on the effectiveness and safety of Gla-300 in wider geographic regions are warranted. The ATOS study aimed to investigate the effectiveness and safety of Gla-300 when added to oral anti-hyperglycaemic drug (OAD) therapy after its failure in people with T2DM in a real-world clinical practice setting in Asia, the Middle East, North Africa, Latin America and Eastern Europe.
Methods
Study Design and Participants
A TOUJEO Observational Study (ATOS) was a 12-month prospective, observational study assessing the real-world clinical effectiveness and safety of Gla-300 in people with T2DM who initiated Gla-300 therapy after OAD failure. Participants were enrolled from 18 different countries in various geographical regions (Colombia, Egypt, India, Indonesia, Israel, Jordan, Kuwait, Lebanon, Mexico, Peru, Philippines, Russian Federation, Saudi Arabia, Singapore, Taiwan, Thailand, Ukraine and the United Arab Emirates). This study was conducted in accordance with the guidelines for Good Epidemiology Practice and the Declaration of Helsinki, and the study is registered at the US National Library of Medicine (Clinicaltrials.gov, number NCT03703869). The study protocol was reviewed and approved by the Independent Interdisciplinary Ethics Committee on Ethical Review for Clinical Studies in accordance with the local regulations in each participating country/study centre. The study applied “new-user” design, enrolling patients at the time they commenced treatment to minimise selection bias. All participants provided written informed consent. Adults (≥ 18 years) with uncontrolled T2DM (HbA1c > 7% and ≤ 11%) on one or more OADs were included following the physician’s decision (in accordance with local guidelines) to add Gla-300 to their ongoing OAD regimen. Participants were insulin naïve before being commenced on Gla-300. Exclusion criteria included age < 18 years, type 1 diabetes, past or present receipt of other injectable anti-hyperglycaemic drugs, any contraindication for Gla-300, pregnancy, breast feeding, drug or alcohol abuse, any concomitant disease with life expectancy of < 1 year and concomitant participation in another trial. After initiation of Gla-300, participants were observed for 12 months.
Study Endpoints
The primary endpoint was the percentage of participants achieving their pre-defined individualised HbA1c target (as determined by their treating physician) at month 6. Secondary efficacy endpoints included the percentage of participants achieving their pre-defined individualised HbA1c target at months 3 and 12 and changes in HbA1c, fasting plasma glucose (FPG) and self-monitored blood glucose (SMBG) from baseline to months 3, 6 and 12. Other study endpoints included changes in insulin dose, body weight and hypoglycaemia incidence and rates [per participant-year (PPY)], as well as incidence of adverse events (AEs).
Treatment and Data Collection
The Gla-300 dose and time of injection were recorded at every visit. Participants’ pre-defined individualised glycaemic goal was set by the treating physician at study entry, and titration was performed by the treating physician at their discretion using locally applicable titration algorithms. Data collection was performed from March 2018 to February 2020. Data were collected at baseline and at 3, 6 and 12 months. HbA1c and FPG values available prior to or at each visit were collected; fasting SMBG was calculated as the mean of the three most recent readings prior to each visit.
Statistical Analyses
Safety analyses were undertaken in the eligible population, which included all participants who provided informed consent, met the inclusion/exclusion criteria and initiated Gla-300 within ±31 days of the start of the study. Efficacy analyses were undertaken in the evaluable population (all eligible participants with an HbA1c assessment at month 6). Descriptive statistics of quantitative effectiveness and safety parameters was used. Least square (LS) mean change from baseline in HbA1c, FPG, fasting SMBG and body weight were assessed using a mixed model for repeated measurements (MMRM).
Results
A total of 4550 participants from Asia, the Middle East, North Africa, Latin America and Eastern Europe were recruited by 363 physicians. Overall, 4422 participants received Gla-300 and were eligible for assessment. Please see Supplementary Fig. S1 for complete participant disposition. The majority (90.2%) of participants continued Gla-300 treatment until the end of the study (month 12). The mean ± standard deviation (SD) age was 57.2 ± 10.8 years, and the majority (73.6%) of participants were < 65 years old; 51.8% of participants were female. At baseline, the mean body weight was 80.7 ± 16.3 kg and body mass index (BMI) was 29.4 ± 5.3 kg/m2 (Table 1). The mean duration of diabetes was 10.2 ± 6.2 years, and HbA1c at baseline was 9.28 ± 1.0%. The mean physician-set individualised HbA1c target was 7.0 ± 0.4%, with the majority (70%) of participants having an HbA1c target of 7–7.5%.
The median duration of OAD use was 9.0 years, and 37.1% of participants were on three or more OADs at baseline. Biguanides were the most commonly used OAD at baseline (88.9% of participants), followed by sulfonylureas (73.0%) and dipeptidylpeptidase-4 (DDP-4) inhibitors (43.5%). Very few changes were observed while considering concomitant use of OADs; biguanides (88.4%) were the most used concomitant OAD taken from first administration of the study drug to the end of the study; other common concomitant OADs were sulfonylureas (71.7%) and DDP-4 inhibitors (43.9%).
Effectiveness Outcomes
HbA1c Target Achievement
Six months after initiating treatment with Gla-300, 25.2% [95% confidence interval (CI) 23.8–26.6%] of participants achieved their pre-defined individualised HbA1c target (primary endpoint), increasing to 44.5% (95% CI 42.9–46.1%) at month 12. At month 6, 65.3% of participants had an HbA1c < 8%, which increased to 81.5% by the end of the study (Fig. 1).
Changes in HbA1c, FPG and SMBG
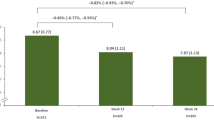
The mean ± SD HbA1c reduced from 9.28 ± 1.0% at baseline to 8.19 ± 1.04%, 7.77 ± 1.06% and 7.38 ± 0.97% at months 3, 6 and 12, respectively, with LS mean change from baseline of −1.07%, −1.50% and −1.87% at months 3, 6 and 12, respectively (Fig. 2). There were no differences in terms of HbA1c change from baseline to month 6 among patients who received concomitant OADs such as sulfonylureas, biguanides, thiazolidinediones, SGLT2 inhibitors or α-glucosidase inhibitors. Incremental reductions were observed in FPG and fasting SMBG over 12 months. The mean ± SD FPG reduced from 11.00 ± 2.96 mmol/L at baseline to 8.07 ± 2.05 mmol/L, 7.54 ± 1.89 mmol/L and 7.01 ± 1.64 mmol/L at months 3, 6 and 12, respectively. The mean ± SD fasting SMBG reduced from 10.76 ± 2.55 mmol/L at baseline to 8.03 ± 1.85 mmol/L, 7.63 ± 1.61 mmol/L and 7.14 ± 1.43 mmol/L at months 3, 6 and 12, respectively (Fig. 2). Treatment intensification with additional concomitant anti-hyperglycaemic drugs were prescribed in 14.3% of participants.
Insulin Dose
At baseline, the mean ± SD starting dose of Gla-300 was 14.6 ± 6.5 units/day (U) corresponding to 0.19 ± 0.09 units/kg body weight/day (U/kg). The mean Gla-300 dose increased from baseline by 4.9 ± 7.1 U (0.06 ± 0.09 U/kg), 7.1 ± 8.5 U (0.08 ± 0.10 U/kg) and 8.6 ± 9.7 U (0.10 ± 0.12 U/kg) at months 3, 6 and 12, respectively, resulting in a mean dose of 19.6 ± 8.6 U (0.25 ± 0.11 U/kg), 21.8 ± 9.5 U (0.27 ± 0.11 U/kg) and 23.3 ± 10.3 U (0.29 ± 0.12 U/kg) at months 3, 6 and 12, respectively. See Table S1 in the supplementary material for details.
Body Weight
There were no significant changes in mean body weight over the 12-month study period. See Supplementary Table S1 for details.
Hypoglycaemia
Incidence and event rates of hypoglycaemia in participants treated with Gla-300 are presented in Table 2. The incidence of documented symptomatic (blood glucose ≤ 3.9 mmol/L) hypoglycaemia was 0.86% at month 6 and 1.27% at month 12. Similarly, the incidence of symptomatic hypoglycaemia represented by blood glucose < 3.0 mmol/L was low at month 6 (0.11%) and month 12 (0.20%). Overall, very few participants reported severe hypoglycaemia at 6 months (0.11%) and 12 months (0.14%).
Safety Outcomes
Overall, treatment-emergent AEs were reported in 283 (6.4%) participants. AEs considered to be possibly related to the use of Gla-300 by the investigator or the sponsor were reported for 12 (0.3%) participants. Serious adverse events (SAEs) were observed in 57 (1.3%) participants. One participant reported an SAE of hypoglycaemic coma, which was considered related to treatment with Gla-300. Fatal AEs were observed in 10 (0.2%) participants; none was considered related to Gla-300. The Medical Dictionary for Regulatory Activities (MedDRA) preferred terms reported, each for one single participant, were sepsis, stage IV gastric cancer, prostate cancer, metastatic small-cell lung cancer, brain oedema, cardiac arrest, coronary artery disease and death of unknown cause; two participants had myocardial infarction. Eight (0.2%) participants had an AE leading to premature treatment discontinuation (Table 3).
Discussion
In this prospective, observational study in Asia, the Middle East, North Africa, Latin America and Eastern Europe, the effectiveness and safety of Gla-300 were evaluated over 12 months in 4422 insulin-naïve people with T2DM. Approximately 25% of participants achieved their pre-defined individualised HbA1c target 6 months after receiving Gla-300; however, this proportion increased to approximately 45% by 12 months. Furthermore, the proportion of participants achieving an HbA1c level of < 7.5% was ~ 41% at month 6 and increased to approximately two-thirds (64%) of participants by month 12. Improvements were also observed for other glycaemic parameters (FPG and SMBG), along with minimal changes in weight and low incidence and event rates of hypoglycaemia. The incidence of AEs was low, with fewer than 1% of participants reporting AEs related to the study treatment, including one SAE. Overall, Gla-300 use was associated with improved glycaemic control and low rates of reported hypoglycaemia in this real-world study population, with minimal change in daily insulin dose and few AEs related to the study treatment.
The results of this real-world study support the findings from other studies of Gla-300 use in insulin-naïve people with T2DM, namely the EDITION 3 and BRIGHT RCTs [17, 19] and the TOUJEO-1, DELIVER Naïve and DELIVER Naïve-D real-world studies [20,21,22]. In brief, the EDITION 3 and BRIGHT studies were treat-to-target RCT of ~ 6 months duration that compared the efficacy and safety of Gla-300 with either the first-generation basal insulin analogue, insulin glargine 100 U/mL (Gla-100) [19], or the second-generation basal insulin, insulin degludec (IDeg) [17]. DELIVER Naïve and DELIVER Naïve-D were real-world retrospective observational studies comparing the effectiveness and safety of Gla-300 with either Gla-100 (DELIVER Naïve) [21] or IDeg (DELIVER Naïve-D) [22] using data from US electronic healthcare records, while the TOUJEO-1 was a prospective 12-month real-world observational study, similar to the ATOS, but was mainly conducted in population from Switzerland and Germany [20].
The proportion of participants reaching their individualised targets in the ATOS (25%) was lower than that of the EDITION 3 (43%) and BRIGHT (49%) RCTs [17, 19]. It should be noted that the EDITION 3 and BRIGHT RCTs used a set HbA1c target of < 7%, rather than individualised targets [17, 19]; however, the mean individualised target set in the ATOS was generally aligned with this goal of 7% (ranging from ≥ 7% to < 7.5% for 70% of the participants). The lower target achievement in the ATOS could be due to more cautious titration in the clinical practice setting (with no recommended titration algorithm in the protocol) of the ATOS compared with the more aggressive treat-to-target titration algorithm used in EDITION 3 and BRIGHT RCTs. In support of this, Gla-300 dose increases from baseline to month 6 observed in the ATOS were much lower than those observed in the EDITION 3 and BRIGHT studies (0.08 U/kg versus 0.43 U/kg and 0.36 U/kg, respectively) [17, 19, 23]. Furthermore, ATOS had a higher baseline HbA1c than that seen in the EDITION 3 and BRIGHT studies (ATOS 9.28%, EDITION 3 8.49% [19] and BRIGHT 8.71% [17]), which might have also contributed to the lower target achievement observed.
The HbA1c target achievement in the ATOS was similar to that seen in the observational DELIVER Naïve (25% at < 7%) [21] and DELIVER Naïve-D (24% at < 7%) [22] real-world studies. However, target achievement in ATOS at 6 months was lower than that observed in TOUJEO-1 (33%) [20]. Of note, this difference in HbA1c target achievement between ATOS and TOUJEO-1 was attenuated at 12 months (ATOS: 45% versus TOUJEO-1: 50%) [20], highlighting that titration in the ATOS might have been gradual but continued over the full 12-month study period.
Interestingly, HbA1c reductions observed at month 6 in ATOS were generally comparable to those observed in the EDITION 3 and BRIGHT RCTs (LS mean changes in HbA1c were −1.50% in ATOS, −1.42% in EDITION 3 and −1.64% in BRIGHT) [17, 19]. Similar results were seen for LS mean FPG reductions (ATOS −3.4 mmol/L, EDITION −3.4 mmol/L [19] and BRIGHT −3.5 mmol/L [17]). This suggests that lower HbA1c reductions may be achieved in real-world studies as in RCTs (as reported previously for studies on anti-hyperglycaemic drugs [24]), perhaps owing to the broader clinical profiles of participants included in real-world studies, along with more rigorous participant compliance in RCTs [25]. This is encouraging given that the FPG targets used for titration in the treat-to-target EDITION 3 and BRIGHT RCTs were likely more stringent than the locally applicable titration targets used in the ATOS. HbA1c reductions in the ATOS at month 6 were also similar to those observed in the DELIVER Naïve (−1.52%) and the DELIVER Naïve-D (−1.67%) real-world studies [21, 22] but were slightly greater than that observed in the TOUJEO-1 study (−1.02%) at month 6 [20]. Similarly, FPG reductions seen in the ATOS were also greater than those reported in the TOUJEO-1 real-world evidence (RWE) study at month 6 (−2.5 mmol/L) [20]. Of note, HbA1c and FPG reductions at 12 months were also greater for the ATOS than for TOUJEO-1 [20].
The high baseline HbA1c and FPG values in the ATOS (HbA1c 9.28%, FPG 11.0 mmol/L) might have contributed to the greater glycaemic reductions than those seen in TOUJEO-1, which had lower baseline values (HbA1c 8.52%, FPG 10.2 mmol/L) [20]. Of note, this might also have contributed to the similar HbA1c reductions seen in the ATOS compared with the EDITION and BRIGHT studies, which also had lower baseline HbA1c and FPG values than the ATOS [17, 19]. Baseline HbA1c values were also high in the DELIVER Naïve and DELIVER Naïve-D studies, which demonstrated similar HbA1c reductions seen in the ATOS [22].
The higher baseline HbA1c and FPG values in the ATOS compared with the EDITION 3 and BRIGHT RCTs are not unexpected, given that these RCTs excluded individuals with HbA1c > 10.5–11% at baseline [17, 19]. While it could be argued that the higher baseline HbA1c and FPG seen in the ATOS compared with the TOUJEO-1 study could be related to a greater insulin treatment inertia in the regions studied in ATOS, it should be noted that the average duration of diabetes in the ATOS (mean 10.2 years) before initiating insulin therapy was comparable to that of EDITION 3 (mean 10.1 years) [19], BRIGHT (mean 10.5 years) [17] and TOUJEO-1 (mean 9.0 years) [20], suggesting this might not be the case. It is also worth noting that other baseline demographics seen in ATOS differed slightly to those seen in studies of the USA and Western European populations. For instance, mean age and mean BMI at baseline were lower in the ATOS (57.2 years and 29.4 mg/kg2) than that in the US and Western-European studies (range of means across studies, age 58.2–64.9 years; BMI 31.2–34.0 mg/kg2) [16, 17, 19,20,21,22], the latter likely being explained by a higher representation of Asian ethnic groups in the ATOS, who are at risk of T2DM at a lower BMI, than the Caucasians [26]. These differences in patient clinical profiles are important to note when comparing the results with other studies [27,28,29,30].
Overall, the incidence and rates of documented symptomatic and severe hypoglycaemia (at night and at any time of the day) were low in ATOS. This supports the well-balanced safety profile of Gla-300 seen in the BRIGHT and EDITION 3 RCTs and in real-world studies of populations in the US and Western Europe [16, 17, 19,20,21,22]. The incidence and rates of reported hypoglycaemia events after 6 months were much lower in the ATOS than that reported in the EDITION 3 and BRIGHT RCTs and the DELIVER Naïve and DELIVER Naïve-D real-world studies [17, 19, 21, 22]. Similarly, 12-month event rates in ATOS were lower than those observed in the LIGHTNING real-world study, which used predictive modelling to estimate hypoglycaemia event rates at 12 months using data from US electronic healthcare records [16]. Possible explanations for the low hypoglycaemia risk seen in the ATOS could be under-reporting due to recall bias or participants not informing their physicians about episodes of hypoglycaemia [31, 32] and the less stringent titration seen in ATOS. Despite higher proportion of participants using sulfonylureas in the ATOS study compared with the TOUJEO-1 study (~ 70% versus ~ 13%, respectively) [20], hypoglycaemia incidence and rates were comparable in the two studies. The high use of sulfonylureas in ATOS might also have influenced the cautious titration observed, as physicians were conscious of avoiding hypoglycaemia; this, in turn, might have impacted the target achievement seen in the ATOS.
Body weight remained stable during the study period in ATOS; this is in contrast with the EDITION 3 and BRIGHT RCTs, which both showed weight increases 6 months following initiation of Gla-300 therapy (~ 0.5 kg and 2.0 kg, respectively) [17, 19]. However, the results of the ATOS were similar to those seen in the TOUJEO-1, which also demonstrated stable bodyweight over time [20]. The fear of weight gain is one of the major barrier to insulin initiation and optimal titration [33]. Consequently, it is encouraging that body weight did not increase in the ATOS; however, these results should be taken in the context of the small Gla-300 dose increments seen.
Strengths of the ATOS study include its real-world design and wide geographical distribution, including countries which may generally be under-represented in terms of diabetes clinical management data. Limitations include the lack of comparator arm; consequently, it is only possible to compare participant data from baseline (before commencing Gla-300) and at months 3, 6 and 12 after commencing Gla-300. Although the lack of comparator is a limitation, the new-user design applied and minimal changes observed in the OAD therapies during the study suggest that the results can be attributed mainly to Gla-300. As with other real-world studies, data on certain outcomes, such as AEs and hypoglycaemia, may be subject to participant recall bias, which could result in underestimation of the true incidence. Furthermore, while real-world studies provide information in a diverse participant population, including high-risk individuals who are often excluded from RCTs may result in confounding factors that could affect results. Participant selection bias may also be present, as the participants were enrolled voluntarily and, therefore, may have adjusted their behaviour owing to their awareness of study monitoring.
Conclusion
In summary, the results observed in the ATOS study support the effectiveness of Gla-300 in a real-world setting and are consistent with the data from RCTs and other RWE studies of Gla-300, as indicated by a pronounced HbA1c reduction from baseline to months 6 and 12 and an increase in the proportion of participants reaching individualised glycaemic targets. Additionally, almost two-thirds of participants reached an HbA1c of 7.5% or less after 12 months. These glycaemic improvements were observed alongside low incidence of reported hypoglycaemia, and stable body weight. However, insulin dose increases were minimal, suggesting suboptimal titration, and in the context of the low incidence of hypoglycaemia seen in ATOS, this observation suggests that more effective titration would have enabled more patients to reach their targets while still maintaining a low risk of hypoglycaemia. Nevertheless, participants initiating therapy with Gla-300 in a real-world setting in Asia, the Middle East, North Africa, Latin America and Eastern Europe still experienced improvements in glycaemic control.
Percentage of participants reaching the pre-defined individualised HbA1c target, HbA1c < 7.5% and HbA1c < 8% at months 3, 6 and 12. *Achievement of individualised HbA1c target at 6 months is the primary endpoint of the study. Efficacy analyses were undertaken in the evaluable population, n = 3704 at month 3, n = 3931 at month 6 and n = 3748 at month 12. #The 3-month period was defined as first treatment administration to visit 2 (month 3); the 6-month period was defined as first treatment administration to visit 3 (month 6) or treatment discontinuation, whichever occurred first, and the 12-month treatment period was defined as first treatment administration to visit 4 (month 12) or treatment discontinuation, whichever occurred first. HbA1c haemoglobin A1c
Mean HbA1c (A), FPG (B) and SMBG (C) levels at baseline and months 3, 6 and 12. Efficacy analyses were undertaken in the evaluable population. Data shown are mean ± SD. †HbA1c: n = 3704 at month 3, n = 3931 at month 6 and n = 3748 at month 12. ‡FPG: n = 3565 at month 3, n = 3718 at month 6 and n = 3579 at month 12. §SMBG: n = 2071 at month 3, n = 2973 at month 6 and n = 2859 at month 12. ¶The 3-month period was defined as first treatment administration to visit 2 (month 3); the 6-month period was defined as first treatment administration to visit 3 (month 6) or treatment discontinuation, whichever occurred first, and the 12-month treatment period was defined as first treatment administration to visit 4 (month 12) or treatment discontinuation, whichever occurred first. LS mean change was assessed using a mixed model for repeated measurements approach. CI confidence interval; FPG fasting plasma glucose, HbA1C haemoglobin A1c, LS least squares, SMBG self-monitored blood glucose
Change history
13 October 2022
A supplementary graphical abstract was retrospectively added to this publication.
References
International Diabetes Federation, Diabetes Atlas 9th Edition 2019. https://www.diabetesatlas.org/en/resources/ (2019). Accessed 2020.
Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11(11):1185–200. https://doi.org/10.7150/ijms.10001.
Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 44 million participants. Lancet. 2016;387(10027):1513–30. https://doi.org/10.1016/S0140-6736(16)00618-8.
Chambers JC, Zhang W, Zabaneh D, et al. Common genetic variation near melatonin receptor MTNR1B contributes to raised plasma glucose and increased risk of type 2 diabetes among Indian Asians and European Caucasians. Diabetes. 2009;58:2703–8. https://doi.org/10.2337/db08-1805.
Shrivastava U, Misra A. Need for ethnic-specific guidelines for prevention, diagnosis, and management of type 2 diabetes in South Asians. Diabetes Technol Ther. 2015;17:435–9. https://doi.org/10.1089/dia.2014.0213.
Shrivastava U, Misra A, Gupta R, Viswanathan V. Socioeconomic factors relating to diabetes and its management in India. J Diabetes. 2016;8:12–23. https://doi.org/10.1111/1753-0407.12316.
Unnikrishnan R, Anjana RM, Mohan V. Diabetes in South Asians: is the phenotype different? Diabetes. 2014;63:53–5. https://doi.org/10.2337/db13-1592.
Unnikrishnan R, Gupta PK, Mohan V. Diabetes in South Asians: phenotype, clinical presentation, and natural history. Curr Diabetes Rep. 2018;18:30. https://doi.org/10.1007/s11892-018-1002-8.
Sutton G, Minguet J, Ferrero C, Bramlage P. U300, a novel long-acting insulin formulation. Expert Opin Biol Ther. 2014;14:1849–60. https://doi.org/10.1517/14712598.2014.970633.
Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859–67. https://doi.org/10.1111/dom.12485.
Ji L, Kang ES, Dong X, et al. Efficacy and safety of insulin glargine 300 U/mL versus insulin glargine 100 U/mL in Asia Pacific insulin-naive people with type 2 diabetes: the EDITION AP randomized controlled trial. Diabetes Obes Metab. 2020;22:612–21. https://doi.org/10.1111/dom.13936.
Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755–62. https://doi.org/10.2337/dc14-0991.
Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235–43. https://doi.org/10.2337/dc14-0990.
Yki-Jarvinen H, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension. Diabetes Obes Metab. 2015;17:1142–9. https://doi.org/10.1111/dom.12532.
Freemantle N, Mauricio D, Giaccari A, et al. Real-world outcomes of treatment with insulin glargine 300 U/mL versus standard-of-care in people with uncontrolled type 2 diabetes mellitus. Curr Med Res Opin. 2020;36:571–81. https://doi.org/10.1080/03007995.2019.1708287.
Pettus J, Roussel R, Liz Zhou F, et al. Rates of hypoglycemia predicted in patients with type 2 diabetes on insulin glargine 300 U/ml versus first- and second-generation basal insulin analogs: the real-world LIGHTNING study. Diabetes Ther. 2019;10:617–33. https://doi.org/10.1007/s13300-019-0568-8.
Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Diabetes Care. 2018;41:2147–54. https://doi.org/10.2337/dc18-0559.
Zhou FL, Ye F, Berhanu P, et al. Real-world evidence concerning clinical and economic outcomes of switching to insulin glargine 300 units/mL vs other basal insulins in patients with type 2 diabetes using basal insulin. Diabetes Obes Metab. 2018;20:1293–7. https://doi.org/10.1111/dom.13199.
Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naive people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386–94. https://doi.org/10.1111/dom.12438.
Pfohl M, Jornayvaz FR, Fritsche A, et al. Effectiveness and safety of insulin glargine 300 U/mL in insulin-naive patients with type 2 diabetes after failure of oral therapy in a real-world setting. Diabetes Obes Metab. 2020;22:759–66. https://doi.org/10.1111/dom.13952.
Bailey TS, Zhou FL, Gupta RA, et al. Glycaemic goal attainment and hypoglycaemia outcomes in type 2 diabetes patients initiating insulin glargine 300 units/mL or 100 units/mL: real-world results from the DELIVER Naïve cohort study. Diabetes Obes Metab. 2019;21:1596–605. https://doi.org/10.1111/dom.13693.
Sullivan SD, Nicholls CJ, Gupta RA, et al. Comparable glycaemic control and hypoglycaemia in adults with type 2 diabetes after initiating insulin glargine 300 units/mL or insulin degludec: the DELIVER Naive D real-world study. Diabetes Obes Metab. 2019;21:2123–32. https://doi.org/10.1111/dom.13793.
Clinicaltrials.gov: Comparison of a new formulation of insulin glargine with Lantus in patients with type 2 diabetes on non-insulin antidiabetic therapy (EDITION III). https://clinicaltrials.gov/ct2/show/results/NCT01676220 Accessed 24 May 2021.
Munk NE, Knudsen JS, Pottegård A, Witte DR, Thomsen RW. Differences between randomized clinical trial participants and real-world empagliflozin users and the changes in their glycated hemoglobin levels. JAMA Netw Open. 2020;3:e1920949-e. https://doi.org/10.1001/jamanetworkopen.2019.20949.
Blonde L, Dendy JA, Skolnik N, White JR Jr. From randomized controlled trials to the real world: putting evidence into context. J Fam Pract. 2018;67:S55-s60.
World Health Organization. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. https://doi.org/10.1016/S0140-6736(03)15268-3.
Mannucci E, Monami M, Dicembrini I, Piselli A, Porta M. Achieving HbA1c targets in clinical trials and in the real world: a systematic review and meta-analysis. J Endocrinol Invest. 2014;37:477–95. https://doi.org/10.1007/s40618-014-0069-6.
Hsieh A, Ong PX, Molyneaux L, et al. Age of diabetes diagnosis and diabetes duration associate with glycated haemoglobin. Diabetes Res Clin Pract. 2014;104:e1-4. https://doi.org/10.1016/j.diabres.2014.02.004.
Home PD, Shen C, Hasan MI, Latif ZA, Chen JW, González GG. Predictive and explanatory factors of change in HbA1c in a 24-week observational study of 66,726 people with type 2 diabetes starting insulin analogs. Diabetes Care. 2014;37:1237–45. https://doi.org/10.2337/dc13-2413.
Yun JS, Ko SH. Risk factors and adverse outcomes of severe hypoglycemia in type 2 diabetes mellitus. Diabetes Metab J. 2016;40:423–32. https://doi.org/10.4093/dmj.2016.40.6.423.
Östenson CG, Geelhoed-Duijvestijn P, Lahtela J, Weitgasser R, Markert Jensen M, Pedersen-Bjergaard U. Self-reported non-severe hypoglycaemic events in Europe. Diabetes Med. 2014;31:92–101. https://doi.org/10.1111/dme.12261.
Emral R, Pathan F, Cortés CAY, et al. Self-reported hypoglycemia in insulin-treated patients with diabetes: results from an international survey on 7289 patients from nine countries. Diabetes Res Clin Pract. 2017;134:17–28. https://doi.org/10.1016/j.diabres.2017.07.031.
Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488–96. https://doi.org/10.1111/dom.13132.
Acknowledgements
The authors are grateful to all study participants and would like to thank all the trial staff and investigators who participated in data collection for the study.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Sanofi, Paris, France.
Medical Writing, Editorial, and Other Assistance
Coordination for the development of this manuscript and assistance with the revisions was provided by Helena Andersson, PhD and Sirisha Pedapudi, MSc, MS of Sanofi. The authors also thank Dimitry Cherkasov (Excelya) and Jany Quan (Sanofi) for analysis and interpretation of the study data. Medical writing and editorial assistance in the preparation of this article was provided by Sambasiva Kolati, MS, who is an employee of Sanofi, and Hannah Brown, PhD of Fishawack Communications Ltd., whose services were funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization and methodology: MAM, MC, MN, VP; investigation: GRG, AT, HVU, NK; formal analysis: MAM, MC, MN, VP; All authors participated in manuscript development and reviewed and approved the final draft for submission.
Prior Publication
Primary results were first presented at American Association of Clinical Endocrinology (AACE) Gulf Chapter Virtual Eighth Annual Clinical Congress, November 5–8, 2020, and Advanced Technologies & Treatments for Diabetes (ATTD), 2–5 June 2021, Virtual.
Disclosures
Gagik R Galstyan, Amir Tirosh, Hernando Vargas-Uricoechea, Niaz Khan are members of the ATOS Steering Committee and have received honoraria in relation to the ATOS study. Amir Tirosh is a member of the National Diabetes Council; Head of the Diabetes Committee of the Israeli Endocrine Society; received honoraria from Sanofi; grant from Medtronic; consulting fees from Sanofi, NovoNordisk, MSD, Merck, AstraZeneca, Medtronic, Dreamed Diabetes; and received personal fees for participation in advisory boards from Sanofi, NovoNordisk, MSD, Merck, AstraZeneca, Bayer. Hernando Vargas-Uricoechea received honoraria from Sanofi and Abbott; personal fees for participation in advisory boards and funding for attending meetings/travel from Sanofi. Maria Aileen Mabunay, Mathieu Coudert, Mubarak Naqvi, Valerie Pilorget are employees and shareholders of Sanofi.
Compliance with Ethics Guidelines
This study was conducted in accordance with the guidelines for Good Epidemiology Practice and principles laid down in the 1964 Declaration of Helsinki by the 18th World Medical Assembly and its later amendments. The study is registered at the U.S. National Library of Medicine (Clinicaltrials.gov Number, NCT03703869). The study protocol was reviewed and approved by the Independent Interdisciplinary Ethics Committee on Ethical Review for Clinical Studies in accordance with the local regulations in each participating country/study centre. All participants provided written informed consent.
Data Availability
Qualified researchers may request access to patient-level data and related documents [including, e.g., the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications]. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Galstyan, G.R., Tirosh, A., Vargas-Uricoechea, H. et al. Real-World Effectiveness and Safety of Insulin Glargine 300 U/mL in Insulin-Naïve People with Type 2 Diabetes: the ATOS Study. Diabetes Ther 13, 1187–1202 (2022). https://doi.org/10.1007/s13300-022-01266-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01266-4