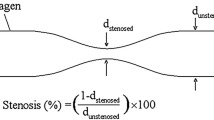
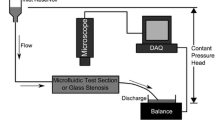
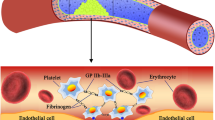
Abstract
Shear stresses play a major role in platelet-substrate interactions and thrombus formation and growth in blood flow, where under both pathological and physiological conditions platelet adhesion and accumulation occur. In this study, a shear-dependent continuum model for platelet activation, adhesion and aggregation is presented. The model was first verified under three different shear conditions and at two heparin levels. Three-dimensional simulations were then carried out to evaluate the performance of the model for severely damaged (stripped) aortas with mild and severe stenosis degrees in laminar flow regime. For these cases, linear shear-dependent functions were developed for platelet-surface and platelet–platelet adhesion rates. It was confirmed that the platelet adhesion rate is not only a function of Reynolds number (or wall shear rate) but also the stenosis severity of the vessel. General correlations for adhesion rates of platelets as functions of stenosis and Reynolds number were obtained based on these cases. Finally using the new platelet adhesion rates, the model was applied to different experimental systems and shown to agree well with measured platelet deposition.










Similar content being viewed by others
Abbreviations
- A ROI :
-
Region of interest area (mm2)
- C i :
-
Concentration of species i (PLT−1 mL, μM, U mL−1)
- D i :
-
Total mass diffusivity of species i (m2 s−1)
- D b,i :
-
Brownian diffusivity of species i (m2 s−1)
- D s,i :
-
Enhanced diffusivity of species i (m2 s−1)
- [H]:
-
Heparin concentration (U mL−1)
- \(k_{{1,{\text{TxA}}_{2} }}\) :
-
Reaction rate constant for inhibition of TxA2 (s−1)
- k rs :
-
Resting platelet-surface adhesion rate (cm s−1)
- k as :
-
Activated platelet-surface adhesion rate (cm s−1)
- k aa :
-
Activated platelet–platelet adhesion rate (cm s−1)
- L :
-
Half width of the channel, tube radius (cm)
- L mar :
-
Platelet margination length scale (m)
- \(M_{\infty }\) :
-
Capacity of surface for first platelet layer (PLT cm−2)
- M as :
-
Surface coverage due to resting platelets (PLT cm−2)
- M as :
-
Surface coverage due to activated platelets (PLT cm−1)
- M at :
-
Deposition due to activated platelets (PLT cm−1)
- N :
-
Average near-wall platelet count (PLT cm−3)
- \(Q_{\text{intial}}\) :
-
Initial flow rate applied at the stenosed channel inlet (mL s−1)
- \(Q_{\text{mean}}\) :
-
Mean flow rate averaged over total perfusion time (mL s−1)
- \(R_{\text{acc}}\) :
-
Number of accumulated platelets per unit time (PLT s−1)
- \(R_{RBC}\) :
-
Radius of red blood cell (m)
- \(Re\) :
-
Reynolds number at inlet
- \(Re_{\text{apex}}\) :
-
Reynolds number at the apex
- \(s_{{p,{\text{TxA}}_{2} }}\) :
-
Rate of synthesis of TxA2 by platelet (nmol PLT s−1)
- S :
-
Available free surface
- \(t_{\text{qss}}\) :
-
Simulation time corresponding to quasi-steady state (s)
- \(V_{\text{acc}}\) :
-
Platelet accumulation rate in region of interest (mm s−1)
- \(\beta\) :
-
Conversion factor to convert thrombin concentration from U mL−1 to μM (nmol U−1)
- \(\dot{\gamma }\) :
-
Local fluid shear rate (s−1)
- \(\dot{\gamma }_{\text{w}}\) :
-
Wall shear rate (s−1)
- \({{\varGamma }}\) :
-
Griffith’s template model for the kinetics of the heparin-catalyzed inactivation of thrombin by antithrombin
- \(\theta\) :
-
Fraction of resting platelets that activate upon surface contact
- \(\lambda_{\text{ADP}}\) :
-
Amount of ADP per activated platelet (nmol PLT−1)
- \(\mu\) :
-
Plasma viscosity (Pa s)
- \(\phi_{\text{rt}}\) :
-
Rate of thrombin generation from prothrombin at the surface of resting platelets
- \(\phi_{\text{at}}\) :
-
Rate of thrombin generation from prothrombin at the surface of activated platelets
- \(\rho\) :
-
Plasma density (kg m−3)
References
Adams, G. A., and I. A. Feuerstein. Maximum fluid concentrations of materials released from platelets at a surface. Am J Physiol Circ Physiol. 244(1):H109–H114, 1983.
Aslan, J., A. Itakura, J. Gertz, and O. T. McCarty. Platelet shape change and spreading. In: Platelets and Megakaryocytes, edited by J. M. Gibbins, and M. P. Mahaut-Smith. New York: Springer, 2012, pp. 91–100. doi:10.1007/978-1-61779-307-3_7.
Badimon, L., and J. J. Badimón. Mechanisms of arterial thrombosis in nonparallel streamlines: platelet thrombi grow on the apex of stenotic severely injured vessel wall. Experimental study in the pig model. J Clin Invest. 84(4):1134, 1989.
Bark, D. L., and D. N. Ku. Wall shear over high degree stenoses pertinent to atherothrombosis. J. Biomech. 43(15):2970–2977, 2010.
Bark, D. L., and D. N. Ku. Platelet transport rates and binding kinetics at high shear over a thrombus. Biophys. J . 105(2):502–511, 2013.
Bark, D. L., A. N. Para, and D. N. Ku. Correlation of thrombosis growth rate to pathological wall shear rate during platelet accumulation. Biotechnol. Bioeng. 109(10):2642–2650, 2012.
Barstad, R. M., H. E. Roald, Y. Cui, V. T. Turitto, and K. S. Sakariassen. A perfusion chamber developed to investigate thrombus formation and shear profiles in flowing native human blood at the apex of well-defined stenoses. Arterioscler. Thromb. Vasc. Biol. 14(12):1984–1991, 1994.
Bluestein, D., E. Rambod, and M. Gharib. Vortex shedding as a mechanism for free emboli formation in mechanical heart valves. J. Biomech. Eng. 122(2):125–134, 2000.
Casa, L. D. C., D. H. Deaton, and D. N. Ku. Role of high shear rate in thrombosis. J. Vasc. Surg. 61(4):1068–1080, 2015.
Casa, L. D. C., and D. N. Ku. High shear thrombus formation under pulsatile and steady flow. Cardiovasc Eng Technol. 5(2):154–163, 2014.
Clemetson, K. J., and J. M. Clemetson. Platelet receptor signalling. Hematol J Off J Eur Haematol Assoc. 5:S159, 2004.
Colace, T. V., and S. L. Diamond. Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow. Arterioscler. Thromb. Vasc. Biol. 33(1):105–113, 2013.
Colace, T. V., R. W. Muthard, and S. L. Diamond. Thrombus growth and embolism on tissue factor-bearing collagen surfaces under flow role of thrombin with and without fibrin. Arterioscler. Thromb. Vasc. Biol. 32(6):1466–1476, 2012.
David, T., S. Thomas, and P. G. Walker. Platelet deposition in stagnation point flow: an analytical and computational simulation. Med. Eng. Phys. 23(5):299–312, 2001.
Duraiswamy, N., J. M. Cesar, R. T. Schoephoerster, and J. E. Moore, Jr. Effects of stent geometry on local flow dynamics and resulting platelet deposition in an in vitro model. Biorheology 45(5):547–561, 2008.
Farokhnia, N., P. Irajizad, S. M. Sajadi, and H. Ghasemi. Rational micro/nanostructuring for thin-film evaporation. J. Phys. Chem. C 120(16):8742–8750, 2016.
Fitzgerald, D. J., and G. A. FitzGerald. Role of thrombin and thromboxane A2 in reocclusion following coronary thrombolysis with tissue-type plasminogen activator. Proc. Natl. Acad. Sci. 86(19):7585–7589, 1989.
Fogelson, A. L. Continuum models of platelet aggregation: formulation and mechanical properties. SIAM J Appl Math. 52(4):1089–1110, 1992.
Fogelson, A. L., and K. B. Neeves. Fluid mechanics of blood clot formation. Annu. Rev. Fluid Mech. 47:377–403, 2015.
Folie, B. J., and L. V. Mcintire. Mathematical analysis of mural thrombogenesis. Concentration profiles of platelet-activating agents and effects of viscous shear flow. Biophys. J . 56(6):1121–1141, 1989.
Frojmovic, M. M., R. F. Mooney, and T. Wong. Dynamics of platelet glycoprotein IIb-IIIa receptor expression and fibrinogen binding. I. Quantal activation of platelet subpopulations varies with adenosine diphosphate concentration. Biophys. J . 67(5):2060, 1994.
Frojmovic, M., T. Wong, and T. van de Ven. Dynamic measurements of the platelet membrane glycoprotein IIb-IIIa receptor for fibrinogen by flow cytometry. I. Methodology, theory and results for two distinct activators. Biophys. J . 59(4):815, 1991.
Goldsmith, H. L., and V. T. Turitto. Rheological aspects of thrombosis and haemostasis: basic principles and applications. ICTH-Report-Subcommittee on Rheology of the International Committee on thrombosis and haemostasis. Thromb. Haemost. 55(3):415–435, 1986.
Goodman, P. D., E. T. Barlow, P. M. Crapo, S. F. Mohammad, and K. A. Solen. Computational model of device-induced thrombosis and thromboembolism. Ann. Biomed. Eng. 33(6):780–797, 2005.
Govindarajan, V., V. Rakesh, J. Reifman, and A. Y. Mitrophanov. Computational study of thrombus formation and clotting factor effects under venous flow conditions. Biophys. J . 110(8):1869–1885, 2016.
Griffith, M. J. Kinetics of the heparin-enhanced antithrombin III/thrombin reaction. Evidence for a template model for the mechanism of action of heparin. J. Biol. Chem. 257(13):7360–7365, 1982.
Hosseinzadegan, H., D. K. Tafti. Validation of a time dependent physio-chemical model for thrombus formation and growth. In: ASME 2016 Fluids Engineering Division Summer Meeting collocated with the ASME 2016 Heat Transfer Summer Conference and the ASME 2016 14th International Conference on Nanochannels, Microchannels, and Minichannels. American Society of Mechanical Engineers, 2016, pp. V01AT04A007.
Hubbell, J. A., and L. V. McIntire. Platelet active concentration profiles near growing thrombi. A mathematical consideration. Biophys. J . 50(5):937, 1986.
Inauen, W., H. R. Baumgartner, T. Bombeli, A. Haeberli, and P. W. Straub. Dose-and shear rate-dependent effects of heparin on thrombogenesis induced by rabbit aorta subendothelium exposed to flowing human blood. Arterioscler. Thromb. Vasc. Biol. 10(4):607–615, 1990.
Irajizad, P., M. Hasnain, N. Farokhnia, S. M. Sajadi, and H. Ghasemi. Magnetic slippery extreme icephobic surfaces. Nat Commun 7:13395, 2016. doi:10.1038/ncomms13395.
Jackson, S. P., W. S. Nesbitt, and S. Kulkarni. Signaling events underlying thrombus formation. J. Thromb. Haemost. 1(7):1602–1612, 2003.
Jones, R. L., N. H. Wilson, C. G. Marr. Thromboxane-like activity of prostanoids with aromatic substituents at C16 and C17. In: Chemistry, Biochemistry, and Pharmacological Activity of Prostanoids: Including the Proceedings of a Symposium on the Chemistry and Biochemistry of Prostanoids Held at The University of Salford, England, 10–14 July 1978. Elsevier, Oxford, 2013, p. 210.
Keller, K. H. Effect of fluid shear on mass transport in flowing blood. In: Federation Proceedings. 1971, pp. 1591–1599.
Lassila, R., J. J. Badimon, S. Vallabhajosula, and L. Badimon. Dynamic monitoring of platelet deposition on severely damaged vessel wall in flowing blood. Effects of different stenoses on thrombus growth. Arterioscler. Thromb. Vasc. Biol. 10(2):306–315, 1990.
Lévêque, A. Les Lois de la transmission de chaleur par convection, par André Lévêque. Paris: Dunod, 1928.
Levich, V. G. Physicochemical Hydrodynamics. Englewood Cliffs: Prentice Hall, 1962.
Li, M., D. N. Ku, and C. R. Forest. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip 12(7):1355–1362, 2012.
Maalej, N., and J. D. Folts. Increased shear stress overcomes the antithrombotic platelet inhibitory effect of aspirin in stenosed dog coronary arteries. Circulation 93(6):1201–1205, 1996.
Mailhac, A., J. J. Badimon, J. T. Fallon, A. Fernandez-Ortiz, B. Meyer, J. H. Chesebro, et al. Effect of an eccentric severe stenosis on fibrin (ogen) deposition on severely damaged vessel wall in arterial thrombosis. Relative contribution of fibrin (ogen) and platelets. Circulation 90(2):988–996, 1994.
Markou, C. P., S. R. Hanson, J. M. Siegel, and D. N. Ku. The role of high wall shear rate on thrombus formation in stenoses. ASME-PUBLICATIONS-BED. 26:555, 1993.
Mehrabadi, M., L. D. C. Casa, C. K. Aidun, and D. N. Ku. A predictive model of high shear thrombus growth. Ann. Biomed. Eng. 44:2339–2350, 2016.
Mehrabadi, M., D. N. Ku, and C. K. Aidun. A continuum model for platelet transport in flowing blood based on direct numerical simulations of cellular blood flow. Ann. Biomed. Eng. 43(6):1410–1421, 2015.
Mehrabadi, M., D. N. Ku, and C. K. Aidun. Effects of shear rate, confinement, and particle parameters on margination in blood flow. Phys. Rev. E 93(2):23109, 2016.
Merrill, E. W. Rheology of blood. Physiol. Rev. 49(4):863–888, 1969.
Mokhlespour, M. I., M. Ramezanzadeh, R. Narimani. Development of a wearable measuring system for respiratory plethysmography. In: Biomed2011, lASTED, Innsbruck, Austria, 2011.
Mokhlespour Esfahani, M. I., S. Taghinedjad, V. Mottaghitalab, R. Narimani, M. Parnianpour, C. Loughlin, et al. Novel printed body worn sensor for measuring the human movement orientation. Sens. Rev. 36(3):321–331, 2016.
Mukherjee, D., N. D. Jani, K. Selvaganesan, C. L. Weng, and S. C. Shadden. Computational assessment of the relation between embolism source and embolus distribution to the circle of willis for improved understanding of stroke etiology. J. Biomech. Eng. 138(8):81008, 2016.
Rosing, J., J. L. Van Rijn, E. M. Bevers, G. Van Dieijen, P. Comfurius, and R. F. Zwaal. The role of activated human platelets in prothrombin and factor X. Blood 65(2):319–332, 1985.
Saelman, E. U. M., H. K. Nieuwenhuis, K. M. Hese, P. G. de Groot, H. F. G. Heijnen, and H. Sage. Platelet adhesion to collagen types I through VI11 under conditions of stasis and flow is mediated by GPIa/IIa (a2Bl-integrin). Blood 83:1244–1250, 1994.
Selvarasu, N. K. C., and D. K. Tafti. Investigation of the effects of dynamic change in curvature and torsion on pulsatile flow in a helical tube. J. Biomech. Eng. 134(7):71005, 2012.
Selvarasu, N. K. C., and D. K. Tafti. Effects of elastic modulus change in helical tubes under the influence of dynamic changes in curvature and torsion. J. Biomech. Eng. 136(8):81001, 2014.
Selvarasu, N. K. C., D. K. Tafti, and P. P. Vlachos. Hydrodynamic effects of compliance mismatch in stented arteries. J. Biomech. Eng. 133(2):21008, 2011.
Soares, J. S., J. Sheriff, and D. Bluestein. A novel mathematical model of activation and sensitization of platelets subjected to dynamic stress histories. Biomech. Model. Mechanobiol. 12(6):1127–1141, 2013.
Sorensen, E. N. Computational Simulation of Platelet Transport, Activation, and Deposition. Pittsburgh: University of Pittsburgh, 2002.
Sorensen, E. N., G. W. Burgreen, W. R. Wagner, and J. F. Antaki. Computational simulation of platelet deposition and activation: II. Results for Poiseuille flow over collagen. Ann. Biomed. Eng. 27(4):449–458, 1999.
Sorensen, E. N., G. W. Burgreen, W. R. Wagner, and J. F. Antaki. Computational simulation of platelet deposition and activation: I. Model development and properties. Ann. Biomed. Eng. 27(4):436–448, 1999.
Taylor, J. O., R. S. Meyer, S. Deutsch, and K. B. Manning. Development of a computational model for macroscopic predictions of device-induced thrombosis. Biomech. Model. Mechanobiol. 15(6):1713–1731, 2016.
Taylor, J. O., L. Yang, S. Deutsch, and K. B. Manning. Development of a platelet adhesion transport equation for a computational thrombosis model. J. Biomech. 50:114–120, 2017.
Turitto, V. T., and H. R. Baumgartner. Platelet interaction with subendothelium in flowing rabbit blood: effect of blood shear rate. Microvasc. Res. 17(1):38–54, 1979.
Vahidi, B., and N. Fatouraee. Large deforming buoyant embolus passing through a stenotic common carotid artery: a computational simulation. J. Biomech. 45(7):1312–1322, 2012.
van der Giessen, A. G., J. J. Wentzel, F. N. van de Vosse, A. F. van der Steen, P. J. de Feyter, F. J. Gijsen. Plaque and shear stress distribution in human coronary bifurcations: a multi-slice computed tomography study. In: ASME 2007 Summer Bioengineering Conference. American Society of Mechanical Engineers, 2007, pp. 477–478.
van der Giessen, A. G., J. J. Wentzel, F. N. van de Vosse, A. F. van der Steen, P. J. de Feyter, and F. J. Gijsen. Plaque and shear stress distribution in human coronary bifurcations: a multi-slice computed tomography study. Am. Soc. Mech. Eng. 36:477–478, 2007.
Varga-Szabo, D., I. Pleines, and B. Nieswandt. Cell adhesion mechanisms in platelets. Arterioscler. Thromb. Vasc. Biol. 28(3):403–412, 2008.
Virchow, R. Gesammelte abhandlungen zur wissenschaftlichen medicin. Frankfurt: Meidinger, 1856.
Vorchheimer, D. A., Becker R. Platelets in atherothrombosis. In: Mayo Clinic Proceedings. Oxford: Elsevier, 2006, pp. 59–68.
Wagner, W. R., and J. A. Hubbell. Local thrombin synthesis and fibrin formation in an in vitro thrombosis model result in platelet recruitment and thrombus stabilization on collagen in heparinized blood. J. Lab. Clin. Med. 116(5):636–650, 1990.
Wang, W., T. G. Diacovo, J. Chen, J. B. Freund, and M. R. King. Simulation of platelet, thrombus and erythrocyte hydrodynamic interactions in a 3D arteriole with in vivo comparison. PLoS ONE 8(10):e76949, 2013.
Weiss, H. J. Platelets: Pathophysiology and Antiplatelet Drug Therapy. New York: AR Liss, 1982.
Weiss, H. J. Flow-related platelet deposition on subendothelium. Thromb. Haemost. 74(1):117–122, 1995.
Wootton, D. M., C. P. Markou, S. R. Hanson, and D. N. Ku. A mechanistic model of acute platelet accumulation in thrombogenic stenoses. Ann. Biomed. Eng. 29(4):321–329, 2001.
Zhao, H., E. S. G. Shaqfeh, and V. Narsimhan. Shear-induced particle migration and margination in a cellular suspension. Phys. Fluids 24(1):11902, 2012.
Acknowledgments
The authors acknowledge the computational support and resources provided by Advanced Research Computing (ARC) at Virginia Tech. This study was funded by the National Science Foundation (Grant CBET-1235790).
Conflict of interest
Hamid Hosseinzadegan declares that he has no conflict of interest. Danesh K. Tafti declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with animals or human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors Baruch Barry Lieber and Ajit P. Yoganathan oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hosseinzadegan, H., Tafti, D.K. Prediction of Thrombus Growth: Effect of Stenosis and Reynolds Number. Cardiovasc Eng Tech 8, 164–181 (2017). https://doi.org/10.1007/s13239-017-0304-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-017-0304-3