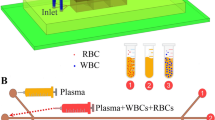
Abstract
White blood cells (WBCs) provide crucial information pertaining to human health, and its separation from other blood constituents is imperative for blood-based diagnostics of various pathological conditions. However, efficient WBC separation and enrichment remain to be a challenge. We propose a novel mechanism of WBC separation in a microfluidic device using centrifugal forces, inertial forces, and diluted human blood prepared with hypertonic saline solution. Herein, a simple wavy type microchannel called the separation channel was designed to separate and enrich WBCs. Almost 88% of WBCs were separated with a purity of 83% with diluted blood, i.e., hematocrit (Hct) 2.8% prepared using a 10% hypertonic solution. In addition, the effect of hematocrit and flow rate on WBC separation is also reported in this work. The proposed microdevice can be used for enrichment and separation of WBCs and also develop a better understanding of the interactions amongst blood cells with altered properties.





Similar content being viewed by others
Abbreviations
- ρ :
-
Density of fluid (kg/m3)
- μ :
-
Dynamic viscosity of fluid (Pa.s)
- d p :
-
Particle/cell diameter (m)
- d h :
-
Hydraulic diameter (m)
- De:
-
Dean Number
- Ref :
-
Flow Reynolds number
- Rech :
-
Channel Reynolds number
- Rep :
-
Particle Reynolds number
- δ :
-
Curvature ratio
- F D :
-
Dean drag force (N)
- F L :
-
Net lift force (N)
- F C :
-
Centrifugal force (N)
- R f :
-
Ratio of lift to drag force
- λ :
-
Confinement ratio
- \({\eta }_{\mathrm{sep}}\) :
-
Separation efficiency
- Ø :
-
Purity/RBC rejection ratio
References
Fung, Y.C.: Biomechanics: mechanical properties of living tissues, vol. 2. Springer Science & Business Media, Berlin (2013)
Caro, C.G.: The mechanics of the circulation, 2nd edn. Cambridge University Press, Cambridge (2011)
Ting-Beall, H.P., Needham, D., Hochmuth, R.M.: Volume and osmotic properties of human neutrophils. Blood 81, 2774–2780 (1993)
Munn, L.L., Dupin, M.M.: Blood cell interactions and segregation in flow. Ann. Biomed. Eng. 36, 534–544 (2008)
Cheng, X., Irimiaa, D., Dixon, M., Sekine, K., Demirci, U., Zamir, L., Tompkins, R.G., Rodriguezb, W., Toner, M.: A microfluidic device for practical label-free CD4+ T cell counting of HIV-infected subjects. Lab Chip 7, 170–178 (2007)
Choi, S., Song, S., Choi, C., Park, J.K.: Continuous blood cell separation by hydrophoretic filtration. Lab Chip 7, 1532–1538 (2007)
Tan, J.K.S., Park, S.Y., Leo, H.L., Kim, S.: Continuous separation of white blood cells from whole blood using viscoelastic effects. IEEE Trans. Biomed. Circuits Syst. 11, 1431–1437 (2017)
Bruil, A., Aken, W.G.V., Beugeling, T., Feijen, J.: Asymmetric membrane filters for the removal of leukocytes from blood. J. Biomed. Mater. Res. 25, 1459–1480 (1991)
Li, X., Chen, W., Liu, G., Lu, W., Fu, J.: Continuous-flow microfluidic blood cell sorting for unprocessed whole blood using surface-micromachined microfiltration membranes. Lab Chip 14, 2565–2575 (2014)
Sethu, P., Anahtar, M., Moldawer, L.L., Tompkins, R.G., Toner, M.: Continuous flow microfluidic device for rapid erythrocyte lysis. Anal. Chem. 76, 6247–6253 (2004)
Nivedita, N., Papautsky, I.: Continuous separation of blood cells in spiral microfluidic devices. Biomicrofluidics 7, 1–14 (2013)
Tay, H.M., Petchacup, C., Dalan, R., Hou, H.W.: Leukocyte fractionation using inertial microfluidics. Conf. Miniaturized Syst. Chem. Life Sci. 367–369 (2015)
Bossuyt, X., Marti, G.E., Fleisher, T.A.: Comparative analysis of whole blood lysis methods for flow cytometry. Cytometry 30, 124–133 (1997)
Zhang, J., Yuan, D., Sluyter, R., Yan, S., Zhao, Q., Xia, H., Tan, S.H., Nguyen, N.T., Li, W.: High-throughput separation of white blood cells from whole blood using inertial microfluidics. IEEE Trans. Biomed. Circuits Syst. (2017). https://doi.org/10.1109/TBCAS.2017.2735440
Sethu, P., Moldawer, L.L., Mindrinos, M.N., Scumpia, P.O., Tannahill, C.L., Wilhelmy, J., Efron, P.A., Brownstein, B.H., Tompkins, R.G., Toner, M.: Microfluidic isolation of leukocytes from whole blood for phenotype and gene expression analysis. Anal. Chem. 78, 5453–5461 (2006)
Wu, Z., Chen, Y., Wang, M., Chung, A.J.: Continuous inertial microparticle and blood cell separation in straight channels with local microstructures. Lab Chip 16, 532–542 (2016)
Laxmi, V., Tripathi, S., Joshi, S.S., Agrawal, A.: Separation and enrichment of platelets from whole blood using a PDMS-based passive microdevice. Ind. Eng. Chem. Res. 59, 4792–4801 (2020)
VanDelinder, V., Groisman, A.: Perfusion in microfluidic cross-flow: separation of white blood cells from whole blood and exchange of medium in a continuous flow. Anal. Chem. 79, 2023–2030 (2007)
Kuan, D.H., Wu, C.C., Su, W.Y., Huang, N.T.: A microfluidic device for simultaneous extraction of plasma, red blood cells, and on-chip white blood cell trapping. Sci. Rep. 8, 1–9 (2018)
Urbansky, A., Olm, F., Scheding, S., Laurell, T., Lenshof, A.: Label-free separation of leukocyte subpopulations using high throughput multiplex acoustophoresis. Lab Chip 19, 1406–1416 (2019)
Han, K.H., Frazier, A.B.: Paramagnetic capture mode magnetophoretic microseparator for high efficiency blood cell separations. Lab Chip 6, 265–273 (2006)
Laxmi, V., Joshi, S.S., Agrawal, A.: Design evolution and performance study of a reliable platelet-rich plasma microdevice. Ind. Eng. Chem. Res. 59, 20515–20526 (2020)
Zhou, J., Papautsky, I.: Size-dependent enrichment of leukocytes from undiluted whole blood using shear-induced diffusion. Lab Chip 19, 3416–3426 (2019)
Jain, A., Munn, L.L.: Biomimetic postcapillary expansions for enhancing rare blood cell separation on a microfluidic chip. Lab Chip 11, 2941–2947 (2011)
Alvankarian, J., Bahadorimehr, A., Yeop, M.B.: A pillar-based microfilter for isolation of white blood cells on elastomeric substrate. Biomicrofluidics 7, 1–16 (2013)
Tasadduq, B., Lam, W., Alexeev, A., Sarioglu, A.F., Sulchek, T.: Enhancing size based size separation through vertical focus microfluidics using secondary flow in a ridged microchannel. Sci. Rep. 7, 1–10 (2017)
Stiles, T., Fallon, R., Vestad, T., Oakey, J., Marr, D.W.M., Squier, J., Jimenez, R.: Hydrodynamic focusing for vacuum-pumped microfluidics. Microfluid Nanofluid 1, 280–283 (2005)
Tripathi, A., Riddell, J., Chronis, N.: A biochip with a 3D microfluidic architecture for trapping white blood cells. Sens. Actuators B: Chem. 186, 244–251 (2013)
Sethu, P., Sin, A., Toner, M.: Microfluidic diffusive filter for apheresis (leukapheresis). Lab Chip 6, 83–89 (2006)
Wilding, P., Kricka, L.J., Cheng, J., Hvichia, G., Shoffner, M.A., Fortina, P.: Integrated cell isolation and polymerase chain reaction analysis using silicon microfilter chambers. Anal. Biochem. 257, 95–100 (1998)
Ji, H.M., Victor, S., Yu, C., Heng, C.K., Lim, T.M., Yobas, L.: Silicon-based microfilters for whole blood cell separation. Biomed. Microdevices 10, 251–257 (2008)
Civin, C.I., Ward, T., Skelley, A.M., Gandhi, K., Peilun, L.Z., Dosier, C.R., D’Silva, J.L., Chen, Y., Kim, M., Moynihan, J., Chen, X., Aurich, L., Gulnik, S., Brittain, G.C., Recktenwald, D.J., Austin, R.H., Sturm, J.C.: Automated leukocyte processing by microfluidic deterministic lateral displacemen. Int. Soci. Adv. Cytometry 89, 1073–1083 (2016)
Davis, J.A., Inglis, D.W., Morton, K.J., Lawrence, D.A., Huang, L.R., Chou, S.Y., Sturm, J.C., Austin, R.H.: Deterministic hydrodynamics: taking blood apart. Proc. Natl. Acad. Sci. 103, 14779–14784 (2006)
Kim, M., Mo, J.S., Lee, K.H., Kang, Y.J., Yang, S.: A microfluidic device for continuous white blood cell separation and lysis from whole blood. Artif. Organs 34, 996–1002 (2010)
Chen, J., Chen, D., Yuan, T., Xie, Y., Chen, X.: Microfluidic chip for direct and rapid trapping of white blood cells from whole blood. Biomicrofluidics 7, 034106 (2013)
Cupelli, C., Borchardt, T., Steinct, T., Paust, N., Zengerle, R., Santer, M.: Leukocyte enrichment based on a modified pinched flow fractionation approach. Microfluid. Nanofluidics 14, 551–563 (2013)
Shevkoplyas, S.S., Yoshida, T., Munn, L.L., Bitensky, M.W.: Biomimetic autoseparation of leukocytes from whole blood in a microfluidic device. Anal. Chem. 77, 933–937 (2005)
Yamada, M., Seki, M.: Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip 5, 1233–1239 (2005)
DiCarlo, D., Irimia, D., Tompkins, R.G., Toner, M.: Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 104, 18892–18897 (2007)
Price, T.H., Dale, D.C.: Blood kinetics and in vivo chemotaxis of transfused neutrophils: effect of collection method, donor corticosteroid treatment, and short-term storage. Blood 54, 977–986 (1979)
Chiu, P.L., Chang, C.H., Lin, Y.L., Tsou, P.H., Li, B.R.: Rapid and safe isolation of human peripheral blood B and T lymphocytes through spiral microfluidic channels. Sci. Rep. 9, 8145 (2019)
Wu, L., Guan, G., Hou, H.W., Bhagat, A.A.S., Han, J.: Separation of leukocytes from blood using spiral channel with trapezoid cross-section. Anal. Chem. 84, 9324–9331 (2012)
Lombodorj, B., Tseng, H.C., Chang, H.Y., Lu, Y.W., Tumurpurev, N., Lee, C.W., Ganbat, B., Wu, R.G., Tseng, F.G.: High-throughput white blood cell (leukocyte) enrichment from whole blood using hydrodynamic and inertial forces. Micromachines 11, 1–12 (2020)
Ozbey, A., Karimzadehkhouei, M., Akgonul, S., Gozuacik, D., Kosar, A.: Inertial focusing of microparticles in curvilinear microchannels. Sci. Rep. (2016). https://doi.org/10.1038/srep38809
De Carvalho, W.B.: Hypertonic solution for pediatric patients. J. Pediatr. 79, 187–194 (2003)
Younes, R.N., Birolini, D.: Hypertonic/hyperoncotic solution in hypovolemic patients: experience in the emergency room. Rev. Hosp. Clin. Fac. Med. Sao. Paulo 57, 124–128 (2002)
Gorter, E., Grendel, F.: On bimolecular layers of lipids on the chromocytes of the blood. J. Exp. Med. 41, 439–443 (1925)
Cooper, G.M.: The cell: a molecular approach, 6th edn. Sinauer, Sunderland (2013)
Jarvela, K., Koskinen, M., Koobi, T.: Effects of hypertonic saline (7.5%) on extracellular fluid volumes in healthy volunteers. Forum Anaesthesia 58, 874–910 (2003)
Han, J., Ren, H.Q., Zhao, Q.B., Wu, Y.L., Qiao, Z.Y.: Comparison of 3% and 7.5% hypertonic saline in resuscitation after traumatic hypovolemic shock. Shock 43, 244–249 (2015)
Carden, M.A., Fay, M.E., Lu, X., Mannino, R.G., Sakurai, Y., Ciciliano, J.C., Hansen, C.E., Chonat, S., Joiner, C.H., Wood, D.K., Lam, W.A.: Extracellular fluid tonicity impacts sickle red blood cell deformability and adhesion. Blood 130, 2654–2663 (2017)
Mortimer, D.S., Jancik, J.: Administering hypertonic saline to patients with severe traumatic brain injury. J. Neurosci. Nursing 38, 142–146 (2006)
Mark, D.S., Kerryn, M., Hongshen, M.: Assessing the vascular deformability of erythrocytes and leukocytes: from micropipettes to microfluidics. Curr. Futu. Aspects Nanomedicin, Iintechopen (2020)
Baker, R.F.: The fine structure of stromalytic forms produced by osmotic hemolysis of red blood cells. J. Ultrastruct. Res. 11, 494–507 (1964)
Dourmashkin, R.R., Rose, W.F.: Morphologic changes in the membranes of red blood cells undergoing hemolysis. Am. J. Med. 41, 699–710 (1966)
Parichehreh, V., Estrada, R., Kumar, S.S., Bhavanam, K.K., Raj, V., Raj, A., Sethu, P.: Exploiting osmosis for blood cell sorting. Biomed. Microdevices 13, 453–462 (2011)
Goodhead, L.K., MacMillan, F.M.: Measuring osmosis and hemolysis of red blood cells. Adv. Physiol. Educ. 41, 298–305 (2017)
Tripathi, S., Prabhakar, A., Kumar, N., Singh, S.G., Agrawal, A.: Blood plasma separation in elevated dimension T-shaped microchannel. Biomed. Microdevices 15, 415–425 (2013)
Choi, H.S., Kim, J.W., Cha, Y.N., Kim, C.: A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J. Immunoassay Immunochem. 27, 31–44 (2006)
Huang, X., Jeon, H., Liu, J., Yao, J., Wei, M., Han, W., Chen, J., Sun, L., Han, J.: Deep-learning based label-free classification of activated and inactivated neutrophils for rapid immune state monitoring. Sensors (2021)
Tripathi, S., Kumar, Y.V.B.V., Agrawal, A., Prabhakar, A., Joshi, S.S.: Microdevice for plasma separation from whole human blood using bio-physical and geometrical effects. Sci. Rep. (2016). https://doi.org/10.1038/srep26749
Yuxi, S., Palaniappan, S.: Low-stress microfluidic density-gradient centrifugation for blood cell sorting. Biomed. Microdevices 20, 77 (2018)
Low, W.S., Wan, A.W.A.B.: Benchtop technologies for circulating tumor cells separation based on biophysical properties. Biomed. Res. Int. (2015)
Al-Faqheri, W., Thio, T.H.G., Qasaimesh, M.A., Dietzel, A., Madou, M., Al-Halhouli, A.: Particle/cell separation on microfluidic platforms based on centrifugation effect: a review. Microfluid. Nanofluidics 21, 1–23 (2017)
Motaharinia, J., Etezadi, F., Moghaddas, A., Mojtahedzadeh, M.: Immunomodulatory effect of hypertonic saline in hemorrhagic shock. DARU J. Pharm. Sci. 23, 1–8 (2015)
Ying, Y., Lin, Y.: Inertial focusing and separation of particles in similar curved channels. Sci. Rep. 16575 (2019)
Martel, J.M., Toner, M.: Particle focusing in curved microfluidic channels. Sci. Rep. 3, 3340 (2013)
Martel, J.M., Toner, M.: Inertial focusing in microfluidics. Annu. Rev. Biomed. Eng. 16, 371 (2014)
DiCarlo, D., Edd, J.F., Irimia, D., Tompkins, R.G., Toner, M.: Equilibrium separation and filtration of particles using differential inertial focusing. Anal. Chem. 80, 2204–2211 (2008)
Tripathi, S., Kumar, A., Kumar, Y.V.B.V., Agrawal, A.: Three-dimensional hydrodynamic flow focusing of dye, particles and cells in a microfluidic device by employing two bends of opposite curvature. Microfluid. Nanofluidics 20, 34 (2016)
Adalgisa, I.M., Giuseppe, A.P., Sebastiao, D.S.F., Severo, D.P., Tania, S.G., Adenilson, S.F., Mario, B.: Osmotic and morphological effects on red blood cell membrane: action of an aqueous extract of Lantana camara. Brazilian J. Pharmacognosy 18, 42–46 (2008)
Hladky, S.B., Rink, T.J.: Osmotic behaviour of human red blood cells: an interpretation in terms of negative intracellular fluid pressure. J. Physiol. 274, 437–446 (1978)
Ponder, E.: The relation between red blood cell density and corpuscular hemoglobin concentration. J. Biol. Chem. 144, 333–338 (1942)
Jain, A., Munn, L.L.: Determinants of leukocyte margination in rectangular microchannels. PLoS ONE 4, 1 (2009)
Mane, S., Hemadri, V., Tripathi, S.: Margination of white blood cells within straight and curved microchannels. ICRAFT2020_3067, 2nd International Conference on Recent Advances in Fluid and Thermal Sciences (ICRAFT), BITS Pilani, Dubai Campus, UAE, March 19-21 (2021)
Boyum, A.: Separation of lymphocytes, lymphocyte subgroups and monocytes: a review. Lymphology 71–76 (1977)
Cossarizza, A. et al.: Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol 1584–1797 (2017)
Li, X., Li, Y., Shao, W., Li, Z., Zhao, R., Ye, Z.: Trategies for enrichment of circulating tumor cells. Transl. Cancer Res. 2012–2025 (2020)
Roberts, R.L., Yohichiroh, O., Gallin, J.I.: Staining of eosinophils with nitroblue tetrazolium in patients with chronic granulomatous disease. Pediatr. Res. 20, 378–380 (1986)
Acknowledgements
We are grateful to Mr. Hemeshwar, CNC operator cum technician from BITS-Pilani, K. K. Birla Goa campus, for assisting us in fabrication of the master mold using the micro-milling process. S.T. acknowledges the financial support received under the Additional competitive grant provided by BITS-Pilani, K. K. Birla Goa campus.
Funding
Birla Institute of Technology and Science, Pilani, Additional competitive grant (GOA/ACG/2019-20/Oct/01).
Author information
Authors and Affiliations
Contributions
SM, VH, and ST designed the research; SM conducted the experiments; SM and ST analyzed and interpreted the data; SM, and ST wrote the manuscript. All the authors reviewed and edited the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
All the experiments were carried out in accordance with the rules and regulations permitted by the Human ethical committee, Bits-Pilani, KK Birla Goa campus. Blood samples in this study were obtained with written informed consent from all subjects. All blood samples were pretested for any communicable diseases.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13206_2022_74_MOESM1_ESM.docx
Supplementary file1 Includes performance parameters, cell counting and videos. Separation of WBCs using 10% NaCl and 2.8% Hct at a flow rate of 130 µl/min (movie/video 1) and Separation of WBCs using 0.9% NaCl and 2.8% Hct at a flow rate of 130 µl/min (movie/video 2) (DOCX 65 KB)
Supplementary file2 (AVI 7020 KB)
Supplementary file3 (AVI 5212 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mane, S., Hemadri, V. & Tripathi, S. Separation of White Blood Cells in a Wavy Type Microfluidic Device Using Blood Diluted in a Hypertonic Saline Solution. BioChip J 16, 291–304 (2022). https://doi.org/10.1007/s13206-022-00074-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-022-00074-z