Abstract
The main purpose of this work is to prepare an efficient series of preformed particle gels (PPGs) and to study their behaviors to control unwanted water in oil fields. To this end, 12 copolymer samples composed of acrylamide and acrylic acid [poly(AAm-co-AA)] with different AAm/AA mole ratios and various mole percentages of N,N′-methylenebisacrylamide (MBA) were synthesized by a free radical copolymerization method. The gelation time of each sample was measured. The samples were dried in three consecutive steps: drying at atmospheric condition, drying in the oven, and drying in the vacuum oven, respectively. The chemical structure and morphology of the prepared PPGs were studied by a Fourier transform infrared spectroscopy and a scanning electron microscopy, respectively. Additionally, the swelling percentage of PPG samples was examined in different types of salt water (MgCl2·6H2O, CaCl2·2H2O, BaCl2·2H2O, NaCl, KCl, and LiCl) with 200,000 ppm concentration and pH in the range of 3–8. Our results showed that the synthesized PPGs had swelling percentage (S%) in the range of 2–1300% for different types of salt solution. Moreover, the results confirmed that the swelling amount of PPG samples has a parabolic behavior against mole ratio of AAm/AA and decreases with increasing mole percentage of MBA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unwanted water production is a crucial issue for most oil producers (Wen et al. 2016; Yu et al. 2017). Corrosion, the load on fluid handling facilities, and environmental problems are increased by increasing the amount of unwanted water production. Even in certain cases, this phenomenon can lead to shut-in well. Fractures are able to establish a connection between an injection well and a production well (Li et al. 2017). Hitherto, several carbonate fractured reservoirs were examined and their fluid properties which influence oil recovery were determined (Abdelazim 2016; Barros-Galvis et al. 2017; Parker-Lamptey et al. 2017; Rahimi et al. 2017; Ruidiaz et al. 2017). However, controlling unwanted water production has been the major purpose of oil and gas producers (Chen et al. 2017; Izadmehr et al. 2017). One of the best cost-effective methods to alleviate this problem is gel treatment in which the major role of gel is to absorb water through highly permeable zones. There are two kinds of gels that absorb water: in situ gels and PPGs. PPGs are adjustable in size from µm to cm and can swell in water and have a controllable swelling rate. They can resist against many kinds of salt solutions with high concentrations and can withstand high pressure and temperature. To do so, oil producers confirm that PPGs are more successful than in situ gels in controlling unwanted water production from oil or gas reservoirs (Bai et al. 1999, 2007b; Bai and Zhang 2011; Tongwa et al. 2013a, b). Since PPGs were more successful than in situ gels in controlling unwanted water, the present paper mainly focused on physical and chemical properties of the synthesized PPG, which is basically attributed to the approach to be utilized in the synthesis process. The synthesis approach of PPG and saltwater properties in which PPG is placed can affect PPG performance (Tongwa and Baojun 2015). Hitherto, many attempts have been made to synthesize PPGs and to examine their behavior under different conditions by many researchers. Isik (2004) synthesized a copolymer of acrylamide and acrylic acid and explored its swelling performance. Different weight ratios of AAm/AA, the amount of polyethylene glycol (PEG) 4000, and mole percentage of N,N′-methylenebisacrylamide on swelling behavior of hydrogels showed that an increase in AA, PEG 4000, and cross-linker concentration results in decreased capacity of hydrogels swelling. Caykara et al. (Çaykara and Akçakaya 2006) synthesized ionic hydrogels from acrylamide (AAm), dimethylamylamine (DMAAm), and itaconic acid (IA) by free radical cross-linking copolymerization approach. Results showed that the swelling behavior of hydrogels at buffer solutions with different pHs can be described by the modified Flory–Rehner equation based on the affine and phantom network models. Liu et al. (2006) synthesized poly(N-isopropylacrylamide) with the use of a special kind of hectorite (Laponite XLS) modified with tetrasodium pyrophosphate. The outcomes demonstrated that these hydrogels have good mechanical properties and complex deswelling behavior which was due to high clay content of hydrogels. Bai et al. (2007a) reported that gelant compositions, temperature, brine salinity, and pH are vital factors affecting PPG properties. Moreover, the results revealed that gel swelling capacity decreases with the increase in salinity unlike swollen gel strength. In addition, temperature effect on the swelling capacity is contrary to salinity. Hussain et al. (2012) produced superabsorbent cross-linked poly(acrylic acid) via suspension polymerization in supercritical CO2. The solubility of acrylic acid in supercritical CO2 has a strong effect on the particle morphology. Ahmed (2015) examined several technologies of hydrogel preparation and additionally considered their physical and chemical properties. Malana et al. (2014) synthesized pH-sensitive hydrogels from acrylamide, methacrylate, and acrylic acid with the use of different cross-linker agents and investigated the effect of pH and the nature of cross-linker agent on the swelling capacity of hydrogels. It was concluded that the network parameters and absorbency changed very sharply with changing pH value. Tongwa et al. (Tongwa and Baojun 2015) synthesized a preformed particle gel with calcium montmorillonite as a nanomaterial and disclosed that the presence of the nanomaterial in hydrogel causes a remarkable improvement in gel properties and its behavior in comparison with hydrogels without any nanomaterial. Pacheco et al. (2014) prepared hydrogels from acrylamide and sodium methacrylate (NMA) by solution polymerization and examined the swelling behavior of hydrogels. Results showed that the weight of sodium methacrylate can greatly affect the swelling behavior of hydrogels and can reduce the dynamic correlation length (ξ). Hamouda et al. (Hamouda and Amiri 2014) reported that the weight percentage of alkaline sodium silicate in reaction solution, low pH value, presence of divalent ions, and temperature reduction can accelerate the gelation time, and the increase in Ca2+ and Mg2+ ion concentration can increase gel strength. Othman et al. (2015) synthesized hyperbranched polyimide derived from melamine by emulsion polymerization. The effect of monomer feed ratio, reaction medium properties, and temperature was studied to find the optimal synthesis conditions. Craciun et al. (2016) outlined the synthesis of acrylamide and acrylic acid in aqueous solutions. Diffusion coefficient and network parameters of hydrogels were investigated, and a possible reaction mechanism was suggested. Zhong et al. (2016) found that the mechanical properties of poly(acrylic acid) hydrogels are significantly attributed to the contents of covalent cross-linker ions and water.
Despite some achievements in using PPGs, no one has studied the synthesis approach of poly(acrylamide-co-acrylic acid), as a good commercial superabsorbent, in all acceptable range of AAm/AA mole ratio (Buchholz and Graham 1998) and mole percentage of N,N′-methylenebisacrylamide (MBA) (Buchholz and Graham 1998). In addition, there is no report in the literature to study all the key factors (type, concentration, and pH of salt solutions and swelling time) affecting the swelling behavior of PPGs in salt solutions. Examining vital factors affecting swelling capacity of PPGs in salt solutions results in optimum conditions with minimum economic costs and maximum working efficiency. To the best of our knowledge, there is no report in the literature to study the drying and swelling behavior of poly(acrylamide-co-acrylic acid) in different types of salt water (MgCl2·6H2O, CaCl2·2H2O, BaCl2·2H2O, NaCl, KCl, and LiCl) with high concentration (200,000 ppm) and pH in the range of 3–8. This study proposes the optimum conditions for having a maximum swelling percentage of PPGs based on overall acceptable range of key factors (AAm/AA mole ratio, MBA mole percentage, swelling time, and pH). Generally, this study gives oil producers a view on the synthesis and swelling behavior of PPGs in high-salinity water (formation water). Knowing how much PPG is required to be injected into the reservoir in order to efficiently control unwanted water production is a vital information for oil producers (Bai et al. 2008; Liu et al. 2006); therefore, the results of this study are useful for oil producers.
In this study, 12 PPG samples were synthesized by free radical copolymerization approach. Then, for the first time, all the important parameters that can affect the water controlling process including AAm/AA mole ratio, MBA mole percentage, types of salt solutions, time of swelling, and pH on swelling capacity of the synthesized PPGs were investigated. In addition, SEM imaging and FT-IR tests were performed to investigate the morphology and structure of PPG samples.
Experimental
Material and instrumentation
Acrylamide (AAm, > 99%), acrylic acid (AA, > 99%), N,N′-methylenebisacrylamide (MBA, > 99%), and potassium persulfate (KPS, > 99%) were obtained from Sigma-Aldrich Chemical Company (Milwaukee, WI). Distilled water was used throughout this study. Several inorganic salts including sodium chloride (NaCl, > 99.5%), lithium chloride (LiCl, > 98%), potassium chloride (KCl, > 99%), magnesium chloride hexahydrate (MgCl2·6H2O, 99%), calcium chloride dihydrate (CaCl2·2H2O, 99%), and barium chloride dihydrate (BaCl2·2H2O, > 99%) were used (Merck) for swelling studies. NaOH (99%, 0.1 M) and HCl (99%, 0.1 M) were used for adjusting pH value (Sigma-Aldrich). The pH meter (Milwaukee pH Meter w/ATC, ± 0.1 error, calibrated with buffer solutions of pH 4, 7 and 10) was used to measure pH of saline water. The weight of samples was measured by the digital balance (Sartorius balance, BP 210 S, German). The filter paper (Whatman filter paper, grade 40:8 µm) was used to dry–wet swollen hydrogel samples. FT-IR spectra of samples were recorded using Shimadzu FT-IR 8300 instrument. The specimens were made ready for IR test by grounding with KBr and subsequent pressing into a pellet. Samples spectra were collected in the range of 4000–500 cm−1. Scanning electron micrographs were derived using scanning electron microscopy (SEM, VEGA3 TESCAN, at 20 kV). Prior to SEM observation, PPG samples are sputter-coated with gold with physical vapor deposition technique (PVD) using sputter coater (VEGA3 TESCAN).
Synthesis of hydrogel
Free radical polymerization was carried out in capped poly(vinyl chloride) straws with an inner diameter of 3 mm. To do so, 0.5 g of AAm and a certain amount of AA were solved in the 1.0 mL of distilled water in the straw. 0.11 mL of the prepared solution of MBA (0.05–0.4 g MBA/mL water) was then added to the straws as the cross-linker agent. In the end, 0.05 mL of the initiator solution (0.05 g KPS/mL water) was subjoined to the reaction mixture. The production conditions of PPG samples are given in Table 1. The straws were placed in an oil bath at 80 °C for 3 h. At last, hydrogels were washed with distilled water to remove unreacted chemicals, cut into small pieces, and were dried until reaching a constant weight.
Gelation time
The tilting method is employed to measure the gelation time. After adding the initiator (KPS) according to the previous section, the viscosity of polymer solution continuously increased during the polymerization process. When the full network structure (gel) obtained and the reactant mixture does not descend in the tilted tube, the gelation time is determined.
Drying synthesized samples
After formation of hydrogels in capped poly(vinyl chloride) straws, samples were removed and washed by distillated water. Evaluating the distillated water confirmed that all monomers were completely reacted. Next, the synthesized samples were dried in three steps. First, water adsorption of hydrogels was dried immediately on filter paper and then samples were put on the digital balance at atmospheric condition and weighed every five minutes for half an hour using the digital balance. After completing the first stage, samples were rapidly put into the oven (60 °C) and weighed every five minutes for half an hour as before. In the end, samples were kept in the vacuum oven (60 °C) and weighed after 24 h.
Swelling study
0.1 g of each synthesized hydrogel was treated with a solution of CaCl2·2H2O, BaCl2·2H2O, MgCl2·6H2O, NaCl, KCl, and LiCl (20 mL, 200,000 ppm) at room temperature. As the salinity of most deep reservoirs is about 200,000 ppm, the concentration of all salt solutions was selected to be 200,000 ppm. Swollen samples were dried with the filter paper and weighed by the digital balance every 3 min to 3 h. The swelling percentage of samples (S%) was calculated from Eq. (1):
where mw and md are the weight of wet gel at time t and dry gel at the beginning of experiment, respectively.
Equilibrium swelling ratio
Hydrogels (0.1 g) were retained in the saline solutions (20 mL, 200,000 ppm) for 6 months to ensure that samples were swollen as much as possible. The final (equilibrium) water uptake over the weight of dry polymer is called equilibrium swelling ratio and is calculated according to Eq. (2):
where ES is the equilibrium swelling ratio in fraction. Ww and Wd denote the weight of swollen and dried sample, respectively.
pH-sensitivity
To investigate pH effect on the swelling behavior of the hydrogels, two salt solutions (20 mL, 200,000 ppm) were prepared from each of BaCl2·2H2O, CaCl2·2H2O, and MgCl2·6H2O salts; pH of each solution was adjusted both on three (with HCl, 0.1 M) and on eight (with NaOH, 0.1 M). For each salt type, 0.1 g of the dried sample was first put in the alkaline solution for half an hour and then was put in the acidic solution. This sample transition from alkaline to acidic was continued every half an hour to three hours, and vice versa. Every three minutes, swelling percentage of samples and pH of solution were recorded by using Eq. (1) and the pH meter, respectively.
Results and discussion
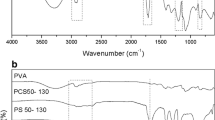
In this study, 12 poly(AAm-co-AA) samples were synthesized by free radical copolymerization approach. As reported (Buchholz and Graham 1998), the mole ratio of AAm/AA and the mole percentage of the cross-linker agent in the copolymerization reaction are better to be in the range of 2–10 mol ratio and 4–20 mol percentage, respectively. In addition, several authors used these mentioned ratios for AAm/AA and MBA (Craciun et al. 2016; Işık 2004; Isık and Kıs 2004). Therefore, in this work, the values of 2.41, 4.82, and 9.65 for AAm/AA mole ratio (0.20, 0.10, and 0.05 mL of AA, respectively) were considered to cover the entire range of AAm/AA mole ratio (Table 1). In addition, the values of 4, 16, 18, and 20 for MBA mole percentage were taken into account to cover the MBA mole percentage range (Table 1). After synthesis experiments, the effect of key parameters (mole ratio of AAm/AA, mole percentage of MBA, swelling time, salt type, and pH) on the swelling behavior of hydrogels was investigated in high-salinity solutions (200,000 ppm). Scheme1, which was drawn with ChemDraw Ultra 7.0 software, shows the reaction occurred in the polymerization medium. To ensure that the desired structures of PPG samples were formed, FT-IR tests were performed. FT-IR spectrum of sample 1 as an example is depicted in Fig. 1. The bands that appeared in the range of 3100–3500 cm−1 (O–H and N–H stretching) confirm the formation of copolymer of acrylamide and acrylic acid. Two absorption peaks appeared around 3394 and 3163 cm−1 are undoubtedly due to the asymmetric and symmetric NH2 stretching vibrations of acrylamide. On the other hand, the broad absorption bands from 3400 cm−1 to 2975 cm−1 can be assigned to the –OH from the carboxylic group. The values at 2916 and 1846 cm−1 were corresponding to the CH2 and CH stretching vibrations, respectively. The C–O stretching vibrations of carbonyl group of acrylamide and acrylic acid occurred at 1643 and 1720 cm−1, respectively. The band observed at 1436 cm−1 is assigned to C–N stretching vibrations, while the peak correlates with C–C stretching emerged at 1118 cm−1.
The morphology of the PPGs was studied by a scanning electron microscopy. It was found that (Fig. S1 (a–d), supplementary information), the most pores existing in samples 1, 3, 5, and 9 had the magnitude of 10, 6, 16, and 1.2 µm, respectively. Therefore, the mole percentage of MBA and mole ratio of AAm/AA can substantially change the size of pores from 1.2 to 16 µm.
Gelation time
Sudden change from a viscous liquid into an elastic structure is called the gelation point and is an isothermal process. This transformation time is defined as the gelation time and depends on two parameters: cross-linker agent concentration and AAm/AA mole ratio. As shown in Fig. 2, increasing mole percentage of MBA increases the viscosity of the hydrogel, whereas at the same time decreases the gelation time. Similarly, increasing AAm/AA mole ratio can restrict the movement of reactants and deactivate the macro-radical growing chains as soon as their formation. Therefore, the viscosity of hydrogel unlike gelation time increases with increased AAm/AA mole ratio.
Drying synthesized samples
At atmospheric condition, surface water and captured water in large pores (10–16 µm) are most likely to evaporate. Figure 3 shows the amount of water evaporated from samples at atmospheric condition. As can be seen, the average amount of evaporated water from samples containing 9.65 and 4.82 mol ratios of AAm/AA was roughly equal. Samples including 2.41 mol ratio of AAm/AA had minimum amount of evaporated water. It reveals that there are more large pores in high AAm/AA mole ratios. As the amount of AAm was constant in all synthesis experiments (0.50 g), there was low amount of AA in high more ratio of AAm/AA. Therefore, higher pores could form in lower amount of AA. Additionally, in high mole ratio of AAm/AA (9.65 mol ratio), the amount of evaporated water from samples decreased with increasing MBA mole percentage, because more cross-connections and small pores are likely to form in high mole percentage of MBA.
After completing the first stage, drying at atmospheric condition, samples were oven dried (60 °C). Every five minutes, hydrogels were removed from the oven and weighted using the digital balance for half an hour. As expected, the oven evaporates water captured in medium pores (6–10 µm). The trend of weight reduction of samples versus AAm/AA mole ratio and MBA mole percentage in the oven was similar to that of atmospheric condition (Fig. S2a, supplementary information). However, the maximum amount and minimum amount of evaporated water were achieved for 9.65 and 2.41 mol ratios of AAm/AA, respectively.
Finally, samples were placed in the vacuum oven for 24 h. In this step, vacuum oven can evaporate all of the captured waters in small pores (1.2 µm). Results showed that the amount of evaporated water from samples with 2.41 mol ratio of AAm/AA was more than other samples. It reveals that there are more small pores in low mole ratio of AAm/AA. With a constant amount of AAm monomers, there are more AA monomers and therefore more small pores in low AAm/AA mole ratio (Fig. S2b, supplementary information).
Swelling study
Ca2+, Mg2+, Ba2+, Na+, K+, Li+, and Cl− are vital cations and inions existing in the formation water and are able to influence the swelling behavior of hydrogels. In this study, the effect of each cation or anion with 200,000 ppm concentration on the swelling behavior of hydrogels was examined. Figure 4 shows the swelling percentage of the PPG samples (S%) in the BaCl2·2H2O salt solution (as an example). As can be seen, the swelling percentage of all hydrogels had an upward trend with increase in time. The swelling percentage of the PPG samples (S%) in other salt solutions was similar to that of the BaCl2·2H2O salt solution (Fig. S3 (a–e), supplementary information). The mole percentage of MBA, concentration of AA, and salt solution type influenced the swelling percentage of PPG, as expressed in detail below.
Effect of MBA concentration
The effect of MBA mole percentage on the swelling percentage of samples 5–8 after 180 min is shown in Fig. 5. As can be seen, increasing the MBA mole percentage leads to decrease the amount of water absorbency. Increasing mole percentage of MBA decreases the spaces among copolymer chains. Therefore, increasing the mole percentage of MBA forms a rigid structure that cannot expand and absorb a large quantity of water. In fact, in all hydrogels, a small increase in MBA percentage causes a significant decrease in swelling capacity. Similar well-known behavior was reported in the literature (Harsh et al. 1990; Peppas and Mikos 1986).
Figure 5 has a power law in swelling behavior with K = 841.52 and n = 0.377 obtained from the curve fitting with Eq. (3):
where n and K are constant values for an individual PPG, which represent the slope and intercept of the swelling percentage logarithmic diagram according to the MBA mole percentage, respectively.
Effect of AAm/AA mole ratio
Swelling percentage of hydrogels (S%) versus AAm/AA mole ratios in all salt solutions is shown in Fig. 6. To focus on the effect of AAm/AA mole ratio, the effective factors including the MBA concentration, swelling time, and AAm weight value were kept at 4 mol%, 180 min, and 0.5 g (as an example), respectively. It was observed that all curves have a maximum point at 4.82 mol ratio of AAm/AA. Therefore, the absorbency first increased with the increase in AAm/AA mole ratio and then it decreased. The initial rise in swelling percentages can be attributed to the existing low amount of AA in high mole ratios of AAm/AA. AAm monomers have a more effective role than AA monomers in high AAm/AA mole ratios. In addition, more hydrogen bonds can be formed between water and amid groups than carbocyclic groups; oxygen is more electronegative than nitrogen. Therefore, swelling percentage increased with increasing AAm/AA mole ratios. The swelling loss after 4.82 mol ratio of AAm/AA may be due to changing copolymer structure into a single polymer structure (polyacrylamide structure). So far, several investigators reported this parabolic behavior (Athawale and Vidyagauri 1998; Lee and Yuan 2000).
Effect of salt solution type
Samples 1 and 5 had more swelling percentage among the examined samples. Previous studies have shown that swelling capacity of “anionic” hydrogels is appreciably affected by ions existing in salt solutions. This undesired phenomenon is due to non-perfect anion–anion electrostatic repulsion resulted from additional cations in the solution. This is well known as “charge screening effect” (Flory 1953; Kwon et al. 1991; Mirdarikvande et al. 2014). In this step, the effect of all existing cations on the swelling percentage of all the samples was examined. As all the examined salt types have chloride ion as anionic species, it can thus conclude that the observed differences in swelling percentage can be attributed to the type of cations (Ca2+, Mg2+, Ba2+, Sr2+, Na+, K+, and Li+). Figure 7a, b represents the results of swelling percentage of samples 1 and 5 in all salt solutions. As can be seen, the ability of water absorbency for hydrogels in salt solutions with divalent cations such as MgCl2·6H2O, CaCl2·2H2O, and BaCl2·2H2O was more than that for monovalent cations such as KCl, LiCl, and NaCl. Three mobile ions can be formed from each mole of divalent salt; therefore, there are more mobile ions in divalent salt solutions than monovalent salt solutions. Thereupon, due to “charge screening effect,” the osmotic pressure and the amount of absorbed water increased in salt solutions with divalent cations.
Equilibrium swelling ratio
Equilibrium swelling ratio is a key factor for oil and gas producers because they would be able to predict how much swelled PPGs should be injected into a reservoir to control unwanted water. The equilibrium swelling ratio in all salt solutions with divalent cations was greater than that with monovalent cations. The equilibrium swelling percentage of all dried PPG samples in all of the salt solutions was obtained based on Eq. (2) and are given in Table 2. It is evident from the results that the maximum equilibrium swelling ratio was found to be 13 for sample 5 swelled in BaCl2·2H2O salt solution.
pH-sensitivity
In this step, three samples 1, 2, and 3 (as an example) and salt solutions having more ability to swell hydrogels (i.e. BaCl2·2H2O, CaCl2·2H2O, and MgCl2·6H2O) were examined. Experiments were conducted from two other points of view, swelling percentage variations of samples and pH variations of salt solutions versus time. Figure 8 shows that PPG samples more swelled over time in the BaCl2·2H2O salt solution in both alkaline and acidic mediums. The trend of two types of the mentioned saline solutions was similar to the BaCl2·2H2O salt solution (Fig. S4 (a and b), supplementary information). In acidic medium, most of the amide functional groups protonate and the main cation–cation repulsive forces between them increased and consequently swelling capacity of samples appreciably increased. At higher values of pH (6–8), some of the carboxylate groups ionized and the repulsion forces between COO− groups cause an enhancement of the swelling capacity. In these experiments, since the samples were first swollen in alkaline medium and then put in the acidic medium, the curves of swelling percentage (S%) of samples which swelled in the acidic medium were higher than those in alkaline medium. Figure 9 shows the pH changes versus time in the BaCl2·2H2O salt solution. As can be seen, pH of all alkaline and acidic mediums was close to pH 4. pH changes in other mentioned salt solutions were similar to those in the BaCl2·2H2O salt solution (Fig. S5 (a and b), supplementary information). In acidic medium, H+ ions are replaced with monovalent cations existing in the polymer chamber. On the other hand, amide functional groups form hydrogen bonds with water. Therefore, the pH of the acidic medium increased over time. In alkaline medium, OH− ions and pH of solutions decreased over time. It was due to increasing hydrogen bonding between carboxylate functional groups with water and, therefore, increasing ionic strength in salt water in accordance with the Debye–Huckel equation (Kwon et al. 1991; Zhong et al. 2016).
Conclusions
In summary, an efficient series of PPGs was produced by a free radical copolymerization method to control unwanted water in mature oil fields. Various samples with different mole percentage of MBA and mole ratio of AAm/AA showed different structures with distinct pores that had an effect on their water absorption. Their gelation time, drying and swelling behaviors (in various salt solutions with different pH values) were examined. Results showed that increasing the MBA mole percentage decreased gelation time, unlike increasing AAm/AA mole ratio, swelling percentage of samples increased by the reduction in MBA mole percentage, the ability of water absorbency first increased with increasing AAm/AA mole ratio and then decreased, and salt solutions with divalent cations such as BaCl2·2H2O, CaCl2·2H2O, and MgCl2·6H2O swelled hydrogels more than those with monovalent cations such as KCl, LiCl, and NaCl. Altogether, findings indicate that synthesized samples had well swelling percentage in high-salinity water (200,000 ppm) in both alkaline and acidic mediums and can be used for water conformance in mature oil fields (e.g., the sample 5 swelled in BaCl2·2H2O salt solution (20 mL, 200,000 ppm) had the equilibrium swelling percentage of 1300%). At last, the optimum conditions for maximum swelling percentage were found to be 4 mol% of MBA, mole ratio of 4.82 for AAm/AA, and 0.05 g/mL of KPS.
References
Abdelazim R (2016) An integrated approach for relative permeability estimation of fractured porous media: laboratory and numerical simulation studies. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-016-0250-x
Ahmed EM (2015) Hydrogel: preparation, characterization, and applications: a review. J Adv Res 6:105–121
Athawale V, Vidyagauri VL (1998) Graft copolymerization onto starch. 3: Grafting of acrylamide using ceric ion initiation and preparation of its hydrogels. Starch-Stärke 50:426–431
Bai B, Zhang H (2011) Preformed-particle-gel transport through open fractures and its effect on water flow. SPE J 16:388–400
Bai B, Li Y, Liu X (1999) New development of water shutoff and profile control in oilfields in China Oil. Drill Prod Technol 20:64–68
Bai B, Li L, Liu Y, Liu H, Wang Z, You C (2007a) Preformed particle gel for conformance control: factors affecting its properties and applications. SPE Reserv Eval Eng 10:415–422
Bai B, Liu Y, Coste J-P, Li L (2007b) Preformed particle gel for conformance control: transport mechanism through porous media. SPE Reserv Eval Eng 10:176–184
Bai B, Huang F, Liu Y, Seright RS, Wang Y (2008) Case study on prefromed particle gel for in-depth fluid diversion. In: SPE symposium on improved oil recovery
Barros-Galvis N, Samaniego VF, Cinco-Ley H (2017) Fluid dynamics in naturally fractured tectonic reservoirs. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-017-0320-8
Buchholz FL, Graham AT (1998) Modern superabsorbent polymer technology. Wiley, New York
Çaykara T, Akçakaya İ (2006) Synthesis and network structure of ionic poly (N,N-dimethylacrylamide-co-acrylamide) hydrogels: comparison of swelling degree with theory. Eur Polym J 42:1437–1445
Chen H, Feng Q, Zhang X, Zhou W, Geng Y (2017) A prediction formula for ratio of injection–production control area in triangle well pattern. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-017-0330-6
Craciun G, Manaila E, Stelescu MD (2016) Electron beam synthesis and characterization of acrylamide/acrylic acid hydrogels using trimethylolpropane trimethacrylate as cross-linker. J Chem. https://doi.org/10.1155/2016/1470965
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, Ithaca
Hamouda AA, Amiri HAA (2014) Factors affecting alkaline sodium silicate gelation for in-depth reservoir profile modification. Energies 7:568–590
Harsh D, Gehrke S, Brannon-Peppas L (1990) Characterization of ionic water absorbent polymers: determination of ionic content and effective cross-link density. Elsevier, Amsterdam
Hussain YA, Liu T, Roberts GW (2012) Synthesis of cross-linked, partially neutralized poly (acrylic acid) by suspension polymerization in supercritical carbon dioxide. Ind Eng Chem Res 51:11401–11408
Işık B (2004) Swelling behavior and determination of diffusion characteristics of acrylamide–acrylic acid hydrogels. J Appl Polym Sci 91:1289–1293
Isık B, Kıs M (2004) Preparation and determination of swelling behavior of poly (acrylamide-co-acrylic acid) hydrogels in water. J Appl Polym Sci 94:1526–1531
Izadmehr M, Daryasafar A, Bakhshi P, Tavakoli R, Ghayyem MA (2017) Determining influence of different factors on production optimization by developing production scenarios. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-017-0351-1
Kwon IC, Bae YH, Kim SW (1991) Electrically erodible polymer gel for controlled release of drugs. Nature 354:291
Lee WF, Yuan WY (2000) Thermoreversible hydrogels X: synthesis and swelling behavior of the (N-isopropylacrylamide-co-sodium 2-acrylamido-2-methylpropyl sulfonate) copolymeric hydrogels. J Appl Polym Sci 77:1760–1768
Li Y-L, Wu F, Li X-P, Tan X-H, Hu X-H, Yang Q (2017) Displacement efficiency in the water flooding process in fracture–vuggy reservoirs. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-017-0321-7
Liu Y, Zhu M, Liu X, Zhang W, Sun B, Chen Y, Adler H-JP (2006) High clay content nanocomposite hydrogels with surprising mechanical strength and interesting deswelling kinetics. Polymer 47:1–5
Malana MA, J-u-D Bukhari, Zohra R (2014) Synthesis, swelling behavior, and network parameters of novel chemically crosslinked poly(acrylamide-co-methacrylate-co-acrylic acid) hydrogels. Des Monomers Polym 17:266–274
Mirdarikvande S, Sadeghi H, Goderzi A, Alahyari M, Shasavari H, Khani F (2014) Effect of pH, and salinity onto swelling properties od hydrogels based on h-alginate-g-poly (AMPS). Biosci Biotechnol Res Asia 11:205–209
Othman MBH, Akil HM, Osman H, Khan A, Ahmad Z (2015) Synthesis, characterisation and thermal properties of hyperbranched polyimide derived from melamine via emulsion polymerisation. J Therm Anal Calorim 120:1785–1798
Pacheco RJ, Silva M, Sousa R, Freitas R (2014) Swelling behavior of polyacrylamide hydrogels near phase transition. Mater Sci Appl 5:610
Parker-Lamptey G, Amoako-Yirenkyi P, Dontwi IK (2017) Fractional radial-cylindrical diffusivity model for levels of heterogeneity in petroleum reservoirs. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-017-0349-8
Peppas N, Mikos A (1986) Preparation methods and structure of hydrogels. Hydrogels Med Pharmacy 1:1–27
Rahimi R, Bagheri M, Masihi M (2017) Characterization and estimation of reservoir properties in a carbonate reservoir in Southern Iran by fractal methods. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-017-0358-7
Ruidiaz E, Winter A, Trevisan O (2017) Oil recovery and wettability alteration in carbonates due to carbonate water injection. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-017-0345-z
Tongwa P, Baojun B (2015) A more superior preformed particle gel with potential application for conformance control in mature oilfields. J Pet Explor Prod Technol 5:201–210
Tongwa P, Nygaard R, Bai B (2013a) Evaluation of a nanocomposite hydrogel for water shut-off in enhanced oil recovery applications: design, synthesis, and characterization. J Appl Polym Sci 128:787–794
Tongwa P, Nygaard R, Blue A, Bai B (2013b) Evaluation of potential fracture-sealing materials for remediating CO2 leakage pathways during CO2 sequestration. Int J Greenh Gas Control 18:128–138
Wen H, Liu Y, Sun N (2016) A new water drive curve at ultra-high water cut stage and application in prediction of oilfield development. J Pet Explor Prod Technol 7:1113–1123
Yu K, Li K, Li Q, Li K, Yang F (2017) A method to calculate reasonable water injection rate for M oilfield. J Petrol Explor Prod Technol 7:1003–1010
Zhong M, Shi F-K, Liu Y-T, Liu X-Y, Xie X-M (2016) Tough superabsorbent poly (acrylic acid) nanocomposite physical hydrogels fabricated by a dually cross-linked single network strategy. Chin Chem Lett 27:312–316
Acknowledgements
The authors are grateful to Shiraz University for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Heidari, S., Esmaeilzadeh, F., Mowla, D. et al. Synthesis of an efficient copolymer of acrylamide and acrylic acid and determination of its swelling behavior. J Petrol Explor Prod Technol 8, 1331–1340 (2018). https://doi.org/10.1007/s13202-017-0428-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-017-0428-x