Abstract
The purpose of this study was to investigate the content and dynamics of nutrients in the shallow (max. 6 m) Lake Mulehe. We collected 54 water samples from nine sampling stations between the wet season (March–May 2020 and dry season (June–August 2020). Nutrients; ammonia–nitrogen (NH4–N), nitrate–nitrogen (NO3–N), nitrite–nitrogen (NO2–N), total nitrogen (TN), total phosphorus (TP) and soluble reactive phosphorus (SRP) were investigated in accordance with APHA 2017 standard procedures. Besides, physical parameters: Temperature, pH, turbidity, electrical conductivity and dissolved oxygen were measured in situ. The water quality index (WQI) was used to determine the water quality of Lake Muhele using drinking water quality standards developed by the Uganda National Bureau of Standards and the World Health Organization. Results indicated that nutrients (TN, NO3–N, TP, NH4-N, NO2–N and SRP) did not differ substantially between study stations (p > 0.05) but did reveal significant differences (p < 0.05) across study months. Besides, nutrient levels differed significantly between seasons (p < 0.05) except for SRP and NH4–N. The WQI values varied from 36.0 to 74.5, with a mean of 58.69. The recorded overall WQI value places Lake Mulehe’s water quality into the ‘poor’ category in terms of worthiness for human consumption. The study, therefore, recommends continuous pollution monitoring and enforcement of local regulations to reduce pollution in the lake as a result of anthropogenic activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fresh water is important not only in natural processes, but also in agriculture, manufacturing operations, and human existence (Duan et al. 2016). Fresh water accounts for only 36 million cubic kilometers of the planet’s total volume of water, which is estimated to be 1.4 billion cubic kilometers (Dunkelman et al. 2018). Nevertheless, anthropogenic activities such as industrialization, urbanization, and extensive agriculture have negatively affected freshwater quality and quantity. Freshwater resources are damaged by waste discharge without proper treatment, according to the United Nations Environment Program (UNEP) (2016). For example, 80 percent of municipal wastewater discharged into water bodies is either improperly treated or untreated as a result of rapid urbanization. The nutrient-rich municipal waste discharge causes eutrophication, which results in a rise in toxic algal blooms, turbidity, and hypoxia in new areas (Mateo-Sagasta et al. 2017; Saturday et al. 2021). The wastewater discharge also poses a health risk to the public as a result of accidental water consumption or skin contact with fecal-contaminated water (Saturday et al. 2021). Disease-causing pathogens present in both human and animal feces, such as bacteria, viruses, protozoa, and worm eggs, may be among the microbial contaminants. Biological and environmental processes require water (Dunkelman et al. 2018). Fresh water is used not only for drinking and bathing but also for recreation, agriculture, energy production, industrial activities and the extraction of fish resources. Besides, considerable number of organisms (i.e., animals, plants, and microbes) rely on freshwater as their natural habitats for survival (Kumar & Afaq 2022).
With approximately 36,280 km2 (15%) of the total land area, Uganda is well endowed with freshwater resources (Failler et al. 2016; Saturday et al. 2021). Surface runoff, urban wastewater effluents and agricultural runoff into freshwater systems, according to the Ministry of Water and Environment (2017), are the biggest risks to the country’s freshwater ecosystems. The rising human population around the Lake Mulehe sub-catchment, combined with agricultural practices have negatively influence the lake’s physiochemical water quality parameters. The fact that Lake Mulehe is situated in a densely populated agricultural area (656.6 people per square kilometer), it is vulnerable to pollution from communities and farmlands. For example, eutrophication could have caused the lake water to change from clear to eutrophic turbid. High algae bloom in a lake system reduces water transparency, depletes DO, and potentially releases toxins, all of which have a detrimental influence on biophysicochemical water quality, as well as altering ecosystem functioning (Ke et al. 2019). Between 1998 and 1999, Magumba (2000) examined the physical, chemical and biological factors of the water quality characteristics of Lake Mulehe among other lakes in the Kigezi sub-region. Since then, no known studies have been conducted on Lake Mulehe to monitor its water quality despite its socioeconomic and ecological importance. Therefore, this study was conducted to investigate the content and dynamics of nutrients in the surface waters of Lake Mulehe to aid in understanding the lake’s ecosystem and long-term management.
Methodology
Study area
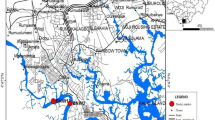
The study was conducted at Lake Mulehe (Fig. 1) located in Kisoro District, Southwestern Uganda. Lake Mulehe is located at 1°13′5" S latitude and 29°43′17" E longitude. The lake is 4.2 km2 in size and has a maximum depth of 6 m and does undergo continuous stratification. Lake Mulehe lies to the East of Lake Mutanda and its water drains through a swamp into Lake Mutanda (Magumba 2000). With considerable subsistence farming, the Lake Mulehe sub-catchment experiences bimodal rainfall. In the lake sub-catchment, the temperature ranges from 19 °C to 25 °C with an average value of 22 °C. Agriculture is the major economic activity, and crops such as beans, sorghum, Irish potatoes, and vegetables are being cultivated. Lake Mulehe is also used for fishing.
Sample collection and analysis
Samples were collected during rainy and dry seasons for a period of 6 months (March–May and June–August 2020) from nine stations (Table 1) giving a total of 54 samples for physico-chemical analysis (Table 1). These stations were selected purposively based on accessibility, proximity to infrastructural developments, lake inflows, and outflows. All the samples were collected below the water surface (at 0.3 m) between 6: 00 and noon. Before the actual collection of samples, the sampling bottles were labeled according to the station codes and rinsed with the lake water four times to remove any possible form of contamination. Samples were collected by dipping 1 L polyethylene plastic bottles below the water surface. Parameters such as Electrical Conductivity, Dissolved Oxygen (DO), pH, turbidity, and water temperature were measured in situ. A DO meter (DO 5510 M.R.C model) was used to measure DO and water temperature, while a water-resistant hand-held pH meter and a conductivity meter were used to measure pH and EC, respectively. A turbidity meter was used to measure the turbidity (2100P, HACH). All measurements were made in triplicate, and the average results were reported. Nutrient parameters such as total nitrogen (TN), total phosphorus (TP), NH4–N, NO2–N and SRP were measured using different methods. TN and TP were determined using the Hach Method 10,072 and HACH Method 8190 method, respectively. NH4–N, NO2–N and SRP concentrations were measured following APHA (2017) standards as described by Saturday et al. (2021). All the nutrient concentrations contained in the samples were recorded directly from a DR 6000 UV Spectrophotometer.
Determination of water quality index
The WQI was calculated based on the relevance of nine physico-chemical parameters in water quality analysis. Physical parameters (i.e., pH, temperature, DO, turbidity and EC) and nutrient parameters (i.e., NO2–N, NO3–N, NH4–N and SRP) were selected for quantification of WQI. The mean value of each station under investigation was used as the value for each parameter. WHO (WHO 2011, 2018) and the Uganda National Bureau of Standards (UNBS 2014) standards for drinking water were used in this study. The relative weights (RW) were calculated by dividing the weight for each variable by the sum of the weights for all variables (Eqs. 1–4). The Weighted Arithmetic Index Method (Brown et al. 1972) was used for the computation of WQI as modified by Saturday et al.(2021).
where
Qn = quality rating of nth water quality parameter
Wn = the unit weight of the nth water quality parameter
Qn was computed using Eq. (2)
where
Vn = the concentration value of nth variable
IV = the ideal value (IV = 0, except for DO (IV = 14.6 mg/L) and pH (IV = 7)
Sn = the standard permissible value for the nth variable.
The unit weight (Wn) was computed using Eq. (3)
where
K = the constant of proportionality was computed using Eq. (4).
Statistical analysis
Statistica software (version 10) was used for statistical data analysis. The mean values of the measured parameters for the study stations were compared to the UNBS (2014) guidelines for natural drinking water sources, the WHO guidelines for drinking water quality, and the USEPA guidelines for recreational waters. To determine whether there were any significant spatial differences among physico-chemical variables, the Kruskal–Wallis test was utilized. Mann–Whitney U test was used to determine whether there were any significant differences between the dry and rainy seasons in terms of measured variables. Spearman’s rank correlation analysis was used to establish whether there were significant relationships among physico-chemical water variables.
Results
Spatial variability of physical water quality parameters and nutrients
Despite slight spatial variability of physical parameters (DO, temperature, turbidity, EC and pH; Table 2), the Kruskal–Wallis test revealed no statistically significant differences (p < 0.05) in any of them between the stations. The same applied to the nutrients (i.e., SRP, TP NH4–N, TN, NO2–N and NO3–N; Table 3). It seems that Rugege (S6) distinguishes itself with the lowest temperature, DO, EC, and pH; highest turbidity and NO2–N. Although all differences between the stations by single parameters were non-significant, the number of extreme values observed at this station is significantly higher.
Temporal variability of physical water quality parameters and nutrients
Unlike pH and water temperature, DO, EC and turbidity values were higher in the wet season than they were in the dry season (Table 4). April 2020 distinguished itself with the highest DO, EC, and pH values; March 2020 recorded the lowest water temperature value of 20.24 ± 0.74 °C (Table 5). Remarkable seasonal differences in physical parameters (i.e., temperature, DO, turbidity and EC; Table 6) were observed (p < 0.05). Nutrients were higher in the wet season than they were in the dry season (Table 7). March 2020 distinguished itself with the highest nutrient concentration (i.e., SRP, TP, TN, and NO3–N) across the study period except for NO2–N with the highest mean value of 0.009 ± 0.001 mg/L recorded in July 2020 (Table 8). Interestingly, nutrients (i.e., SRP, TP, TN, NO2–N and NO3–N) exhibited significant seasonal differences (p < 0.05) except for NH4–N (Table 9).
The relationship between physical parameters and nutrient levels
To establish whether there were significant relationships between physical parameters and nutrient levels observed in Lake Mulehe, Spearman’s correlation was used (Table 10). Nutrients (i.e., TN, TP, NO3–N and SRP) revealed a significant positive correlation with EC and turbidity (Table 10). The NH4–N revealed a positive correlation with other nutrient variables except for TN (Table 10). There were dominating negative correlations of most variables with temperature (8 out of 10) and dominating positive correlations with SRP (9 out of 10). EC recorded significant correlations (5 out of 10) with other variables, whereas pH had only 1. The DO level was significantly positively correlated with NO3–N, TN, SRP, turbidity and EC but significantly negatively correlated with NO2–N (p < 0.05) (Table 10).
Water quality index
The WQI was computed using the values of nine physico-chemical variables (Table 11) that were chosen because of their significance in water quality analysis. The mean value of the stations under investigation was utilized for each variable. The WQI values were calculated using WHO (WHO 2011, 2018) threshold limit values, except for SRP and NH4–N values derived from the Uganda National Bureau of Standards (UNBS 2014) (Table 11). The WQI values in the lake ranged from 36.0 to 74.7 at the Safari Lodge and Nyagasonga stations, respectively, with an overall mean value of 58.7 (Table 12). Except for the Safari Lodge station, which reflected that water from Lake Mulehe is good for drinking, recreation, and domestic purposes due to physico-chemical factors, the recorded WQI range values lie between the “Poor” and “Good” class (Table 12).
The water quality index is a measurement of water quality on a scale. As shown in Table 13, a WQI of 0–25 indicates “Excellent” water quality, 25–50 indicates “Good” water quality, 51–75 indicates “Poor” water quality, 76–100 indicates “Very poor” water quality, and > 100 indicates that the water is “Unsuitable” for drinking (Noori et al. 2019).
Discussion
Variability in physical water quality parameters
A mean DO concentration of 6.55 ± 1.08 mg/L could indicate that in this regard water in Lake Mulehe is suitable for aquatic life. Keister et al. (2020) noted that DO values greater than 2 mg/L support aquatic animals and ecosystems. Similarly, Ghebremedhin and Gupta (2023) reported closely related DO values (6.72 ± 0.22 mg/L) on Lake Chamo in Ethiopia. Tiémoko et al. (2020) reported a DO content range of 4.6–7.7 mg/L for Lake Taabo and Kossou of Ivory Coast, which is consistent with the current study. DO, an indicator of water quality in the aquatic ecosystem is affected by a variety of parameters including microbial activity, temperature, organic matter content, pressure, and sampling time (Das Kangabam & Govindaraju 2019; Mutlu 2019). Furthermore, DO concentration has been linked to spatiotemporal variations of phytoplankton biomass (Saturday et al. 2022), and its presence is required for the survival of complex sorts of biological life in water (Lung’ayia et al. 2022).
Water temperature was found to be within the WHO recommended limit of 25 °C (WHO 2008). Low temperatures recorded in the lake’s ecosystem were attributed to the water mass that enters Lake Mulehe through River Nyamunyuka which drains from the hills of Kanaba Sub-County in Kisoro District. Besides, the lake is situated between steep hills which limits the amount of sunlight heating the surface waters of the lake. In comparison with other previous studies, the recorded temperature range values are closely related to that of Magumba (2000) (i.e., 17.4–2 °C) on Lake Mulehe. In other related studies, Tibihika et al. (2016) and Saturday et al. (2021) reported the overall mean water temperature of Lake Bunyonyi of 22 °C and 21.3 ± 1.4 °C, respectively, which are closely related to the present study findings.
The EC values recorded in the present study did not exceed the WHO maximum permissible limits of 2500 μS/cm stated in global national drinking water guidelines. Therefore, the study findings correctly show that the water in Lake Mulehe is not highly ionized and has a low ionic concentration. Since EC is a function of total dissolved solids (ions concentration), high EC values at the Safari Lodge station can be attributed to high levels of total dissolved solids, whereas low EC values at the Kabahunde station imply untainted lake environment. The rising EC levels of the lake could be a result of surface runoff from agricultural activities and wastes from Lake Mulehe sub-catchment. Previous studies have linked surface runoff, effluents, minerals, and salts from municipal runoff after heavy rainfall to greater levels of electrical conductivity in receiving freshwater bodies (Lung’ayia et al. 2022; Saturday et al. 2021).
Turbidity values did not exceed the maximum limit (20 NTU) for international drinking water guidelines (WHO 2018). Nonetheless, turbidity mean values reported during the rainy season (March–May 2020) were much higher, possibly due to increased phytoplankton biomass and high surface runoff into the lake system. Saturday et al. (2021) observed turbidity mean values ranging between 2.8 ± 0.6 and 4.3 ± 1.6 NTU, which are slightly lower than the values obtained in the current study. Contrary to the present study results, Umer et al. (2020) reported a turbidity range of 28.5–63.0 NTU in Lake Beseka of Ethiopia which was significantly higher than the values recorded in the present study. High turbidity in freshwater lakes prevents light from reaching the water column, delaying phytoplankton and macrophyte development and decreasing primary productivity and oxygen release (Lung’ayia et al. 2022; Saturday et al. 2022; Umer et al. 2020).
The recorded pH range (7.34 ± 0.48–8.15 ± 0.54) suggested a neutral to the alkaline state of the lake ecosystem, which was within the acceptable range (6.5–8.5) for aquatic species to thrive (WHO 2018). Closely related values (7.1–8.8) had earlier been reported by Magumba (2000) on Lake Mulehe. The huge intake of fresh water from River Nyamunyuka and other minor streams that pour into the lake is possibly a response to the observed pH variations. Ongom et al. (2017), Song et al. (2020) and Muduli and Pattnaik (2020) all obtained pH ranges suggesting neutral to slightly alkaline lake conditions, which are consistent with our findings. In contrast to the pH range stated, Niyoyitungiye et al. (2019) found that the optimal pH range below pH 6.5 affects the growth of some aquatic species, while pH values over pH 6.5 affect the ability of some organisms to maintain their salt balance, which can cause a delay in reproduction.
Variability of nutrient concentrations
Total phosphorus (TP) includes both inorganic and organic forms. Because of its extended residence duration in lakes, TP is regarded as the most important nutrient. Opiyo et al. (2019) showed higher TP readings (3.09 ± 0.09 mg/L) in the wet season than in the dry season, which is similar to the findings of the current study. The measured TP levels are the result of lake contamination caused by intensive agriculture in Lake Mulehe sub-catchment. Phosphorus-producing human activities have considerable impact on freshwater ecosystems (Havens & Nürnberg 2004). Lake Mulehe’s location, surrounded by high topography, favors nutrient enrichment due to the high velocity of run-off draining into the lake and its subsequent lengthy stay in the lake.
The spatial difference in TP levels is explained by the proximity of some stations to the lakeshore and the intensity of the nearby agricultural activities. Seasonal rainfall variability in the lake area also explained temporal variations in TP levels. This, in turn, determines the intensity of nutrient deposition, with the wet season experiencing high nutrient enrichment from high surface runoff that sweeps the various nutrients from densely farmed agricultural fields, as contrasted to the dry season, which experiences little to no rain. Saturday et al. (2021) reported an average mean TP value of 0.141 ± 0.168 mg/L in Lake Bunyonyi, which is consistent with the current findings. Contrary to the present study findings, Opiyo et al. (2019) found an average mean TP value of 2.9 ± 0.08 mg/L in Lake Simbi of Kenya, higher than the observed TP values in the current study, owing to multiple years of nutrient inputs from the lake sub-catchment, which is heavily irrigated.
Total nitrogen (TN) is a measure of both organic and inorganic nitrogen levels (Saturday et al. 2021). The role of nitrogen in freshwater systems is determined by the relative amount of various nitrogen forms present. Like TP, high surface run-off into Lake Mulehe is partially responsible for the observed TN levels in the lake system. Besides, the intensity of agricultural practices around the lake under study could also explain the observed spatial differences. The upper Lake Mulehe stations recorded relatively higher TN concentration levels due to runoff from agricultural activities and tourism recreation facilities that enter the lake via Bubuye and Safari Lodge stations, respectively. This backs the popular belief that agriculture in the lake watershed is a major source of nitrogen in freshwater lakes (Huang et al. 2017). Like TP, high surface runoff into Lake Mulehe is partially responsible for the observed TN levels in the lake system. Besides, the intensity of agricultural practices around the study lake could explain the observed spatial differences in TN concentration.
In contrast with the dry season which receives little or no rain, high TN values in the wet season were linked to high rainfall intensity which affects nutrient deposition through surface runoff that sweeps nutrients from crop farms and fecal matter from rural populations around the lake. Saturday et al. (2021) and Ozguven and Demir Yetis (2020) reported TN concentration content values of 1.9 ± 1.9 mg/L in Lake Bunyonyi and 4.9 ± 16.0 mg/L in Big Soda Lake Van of Turkey, respectively, in similar tour study findings. Contrary to the current study findings, Zhou et al. (2020) reported an overall mean TN value of 0.88 ± 0.05 mg/L for Lake Qiandaohu in China, higher than the current study findings.
Ammonia–nitrogen (NH4–N) levels were higher than the recommended limit of 0.025 mg/L for freshwater settings, over which it is harmful to freshwater life (EPA 2001). Tilahun and Ahlgren, (2010) found 0.09 ± 0.08 mg/L in Ethiopia’s Lake Chamo. The observed NH4–N range values are higher than 0.0–4.1 µ/L reported by Magumba (2000) on Lake Mulehe perhaps due to the remineralization of submerged macrophytes. The range of NO2–N values was within the WHO drinking water cutoff value of 0.9 mg/L (WHO 2011). As a result, Lake Mulehe’s water is less prone to create health issues. The NO2–N range values (0.0–4.6 µ/L) reported by Magumba (2000) on Lake Mulehe correlate with values reported in the current study. Nonetheless, the NO2–N values recorded were higher than 1.3 ± 0.7 μg/L recorded by Keyombe and Waithaka (2019). Because of chemical reactions with organic compounds, the presence of nitrites in freshwater lakes can lead to the creation of nitrosamines, which can induce carcinogenic effects (WHO 2017).
The observed NO3–N values did not exceed 11 mg/L, which is within the WHO’s recommended drinking water limits (WHO 2011). NO3–N is an important source of nitrogen for protein synthesis. Inorganic nitrogen is the most common form of nitrogen in natural waters, according to Lodh et al. (2014), and it is the most necessary nutrient for hydrophytes and aquatic algae to grow swiftly. The highest NO3–N concentration measurement (0.01 ± 0.004 mg/L) was found at the Bwidisha station, which could be attributed to farmlands nearby. Tibihika et al. (2016), Maryam et al. (2020) and Tibebe et al. (2019) obtained average NO3–N levels of 33.8 ± 2.1 g/L, 0.46 mg/L and 0.21 mg/L respectively, slightly higher the values obtained in the current study. Although NO3–N is an important nutrient for the growth of aquatic plants in aquatic habitats, a concentration > 90 mg/L is harmful to aquatic species (Amić and Tadić, 2018). Rain, fog, snow, decomposition of organic matter, and fertilizer application in agricultural fields are the key processes that naturally enhance NO3–N content in lake ecosystems.
The temperature was positively connected with NO2–N but inversely correlated with SRP, TN, NO3–N, and SRP, according to the correlation analysis. In Loktak Lake, India, Kangabam et al. (2017) found a significant negative relationship between temperature and NO3–N concentration values. Saturday et al. (2021) found a substantial positive association between TN, NO3–N, and NO2–N levels in Lake Bunyonyi, Uganda. The positive correlation between DO and turbidity had earlier been reported by Bhattrai et al.(2017) in Lake Hwajinpo, South Korea. The significant correlation between water temperature and DO levels differed slightly from those of Ali and Khairy (2016), who found an increase in DO levels when water temperatures were lower due to gas solubility, which increases when temperatures and metabolic activity of organisms are low and decreased, respectively. Temperature, DO, turbidity, NH4–N, TP, and SRP were all favorably associated with the observed positive correlation values. Yu et al. (2020) observed a positive correlation of TN with TP and NH4–N, but a substantial negative correlation with NO2–N which is similar to the present study findings.
Water quality index of Lake Mulehe
The WQI of Lake Mulehe fell into the ‘poor’ category of WQI classification attributed to anthropogenic activities, rather than lithological sources. Some studies had earlier reported similar results. For instance, James et al. (2019) reported a WQI range of 51.9–101.1 indicative of poor water quality in the Nyando River of Kenya. Jindal and Wats (2022) reported a WQI value range of 59.7–83.5 at Sukhna Lake of Chandigarh in India. On the contrary, Shah and Joshi (2017) reported that the water quality at Station 1 of the River Sabarmati in India was good, with a WQI ranging from 19.84 to 44.58 between 2005 and 2008. Nihalani and Meeruty (2020) found a WQI range for River Mahi (30–50) and River Narmada (28–52) in India significantly different to the WQI range reported in the current study. Although Lake Mulehe’s WQI values indicate poor quality, values in the lower lake site indicated deteriorating water quality. Pollutant influx from the upper and middle Mulehe sites to the lower site may have resulted in slightly higher WQI values. Excessive use of fertilizers and chemicals, including pesticides could have caused increased pollution at the Gatare and Nyagasonga as a result of expanded agricultural practices (such as Irish potato production).
Conclusion
Both statistical analyses and WQI were used to assess the content and dynamics of nutrients in Lake Mulehe. All nutrient variables did not differ substantially between study stations but did reveal significant differences across study months. This is attributed to seasonal rainfall variability which in turn determines the intensity of nutrient deposition. The wet season experienced high nutrient enrichment from surface runoff that swept nutrients from densely farmed fields in contrast to the dry season, which experienced little rain. The WQI values varied from 36.0 (good quality) to 74.5 (poor quality), with a mean value of 58.69 (poor quality) implying the ‘poor’ water category in terms of worthiness for human consumption. Nevertheless, water from Lake Mulehe can be used for recreational and agricultural activities. These findings provide a point of reference for policymakers when it comes to establishing standards for effective lake management. Since the current study focused on physico-chemical water quality parameters, there is a need to assess the lake water’s suitability for human consumption using fecal indicator bacteria. Besides, continuous pollution monitoring and enforcement of local regulations to reduce pollution are recommendable.
Data availability
All data generated and analyzed during this study are included in this published article.
Abbreviations
- APHA:
-
American Public Health Association
- DO:
-
Dissolved oxygen
- mg/L:
-
Milligrams per liter
- NH4–N:
-
Ammonia–nitrogen
- NO2–N:
-
Nitrite–nitrogen
- NO3–N:
-
Nitrate–nitrogen
- NTU:
-
Nephelometric turbidity unit
- NWSC:
-
National Water and Sewerage Corporation
- TN:
-
Total nitrogen
- TP:
-
Total phosphorus
- UNBS:
-
Uganda National Bureau of Standards
- USEPA:
-
The United States Environment Protection Agency
- WHO:
-
World Health Organization
- WQI:
-
Water quality index
References
Ali EM, Khairy HM (2016) Environmental assessment of drainage water impacts on water quality and eutrophication level of Lake Idku. Egypt Environ Pollut 216:437–449
Amić A, Tadić L (2018) Analysis of basic physical-chemical parameters, nutrients and heavy metals content in surface water of small catchment area of Karašica and Vučica rivers in Croatia. Environments 5(2):20
APHA (2017) Standard methods for the examination of water and wastewater (23rd ed). American Public Health Association. www.standardmethods.org
Bhattrai BD, Kwak S, Choi K, Heo W (2017) Assessment of long-term physicochemical water quality variations by PCA technique in Lake Hwajinpo, South Korea. J Environ Prot 8(13):1636
Brown RM, McClelland NI, Deininger RA, O’Connor MF (1972) A water quality index—crashing the psychological barrier. In: Thomas WA (ed) Indicators of environmental quality. Springer US, Boston, MA, pp 173–182. https://doi.org/10.1007/978-1-4684-2856-8_15
Das Kangabam R, Govindaraju M (2019) Anthropogenic activity-induced water quality degradation in the Loktak Lake, a Ramsar site in the Indo-Burma biodiversity hotspot. Environ Technol 40(17):2232–2241
Duan W, He B, Nover D, Yang G, Chen W, Meng H, Zou S, Liu C (2016) Water quality assessment and pollution source identification of the eastern Poyang Lake basin using multivariate statistical methods. Sustainability 8(2):133
Dunkelman A, Kerr M, Swatuk LA (2018) The new green revolution: enhancing rainfed agriculture for food and nutrition security in eastern Africa. In: Swatuk LA, Cash C (eds) Water, energy, food and people across the global south: ‘The Nexus’ in an Era of climate change. Springer International Publishing, Cham, pp 305–324. https://doi.org/10.1007/978-3-319-64024-2_12
EPA (2001) Parameters of water quality–interpretation and standards. Wexford. EPA. ISBN, 133
Failler P, Karani P, Seide W (2016) Assessment of the environment pollution and its impact on economic cooperation and integration initiatives of the IGAD region; national environment pollution report—Uganda. IGAD-Inter Governmental Authority on Development
Ghebremedhin SG, Gupta BS (2023) Spatio-temporal water quality assessment and pollution source apportionment of Lake Chamo using water quality index and multivariate statistical techniques. Eur J Environ Earth Sci 4(1):11–19
Havens KE, Nürnberg GK (2004) The phosphorus-chlorophyll relationship in lakes: potential influences of color and mixing regime. Lake Reserv Manage 20(3):188–196
Huang J, Xu C, Ridoutt BG, Wang X, Ren P (2017) Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J Clean Prod 159:171–179
James O, Achieng’Atina GO, Shikuku V, Okowa G (2019) Assessment of water quality of the Nyando River (Muhoroni-Kenya) using the water quality index (WQI) method
Jindal R, Wats M (2022) Evaluation of surface water quality using water quality indices (WQIs) in Lake Sukhna, Chandigarh India. Appl Water Sci 12(1):1–14
Kangabam RD, Bhoominathan SD, Kanagaraj S, Govindaraju M (2017) Development of a water quality index (WQI) for the Loktak Lake in India. Appl Water Sci 7(6):2907–2918
Ke Z, Xie P, Guo L (2019) Ecological restoration and factors regulating phytoplankton community in a hypertrophic shallow lake, Lake Taihu. China Acta Ecol Sinica 39(1):81–88
Keister JE, Winans AK, Herrmann B (2020) Zooplankton community response to seasonal hypoxia: a test of three hypotheses. Diversity 12(1):21
Keyombe J, Waithaka E (2019) Analysis of some aspects of the water quality of Lake Naivasha. Int J Chem Lifesci 6(1):2001–2005
Kumar G, Afaq U (2022) Freshwater biodiversity: importance, threats, and management. In: Mushtaq B, Bandh SA, Wani SA, Mir MF (eds) Biodiversity of freshwater ecosystems: threats, protection, and management. Apple Academic Press, Boca Raton, pp 1–20. https://doi.org/10.1201/9781003277125-1
Lodh R, Paul R, Karmakar B, Das MK (2014) Physicochemical studies of water quality with special reference to ancient lakes of Udaipur City, Tripura, India. Int J Sci Res Publ 4(6):1–9
Lung’ayia H, Wangila B, Masaba J (2022) Water quality status of a stream receiving fish pond discharge using physicochemical indicators in Lake Victoria catchment, Kenya
Magumba MK (2000) Physical, chemical, algal composition and primary production in the four Kisoro minor lakes
Maryam AB, Ovie EA, Gambo J (2020) Monitoring and exploring the spatio-temporal variation of physico-chemical variables of river Hadejia, Nigeria; using statistical approach. Asian J Geograph Res. https://doi.org/10.9734/ajgr/2020/v3i130100
Mateo-Sagasta J, Zadeh SM, Turral H, Burke J (2017) Water pollution from agriculture: a global review. Executive summary. Rome, Italy: FAO Colombo, Sri Lanka: International water management
Ministry of Water and Environment (2017) Water and environment sector performance report 2017. Government of Uganda
Muduli PR, Pattnaik AK (2020) Spatio-temporal variation in physicochemical parameters of water in the Chilika Lagoon. In: Max Finlayson C, Rastogi G, Mishra DR, Pattnaik AK (eds) Ecology, conservation, and restoration of Chilika Lagoon, India. Springer International Publishing, Cham, pp 203–229. https://doi.org/10.1007/978-3-030-33424-6_9
Mutlu E (2019) Evaluation of spatio-temporal variations in water quality of Zerveli stream (northern Turkey) based on water quality index and multivariate statistical analyses. Environ Monit Assess 191(6):335. https://doi.org/10.1007/s10661-019-7473-5
Nihalani S, Meeruty A (2021) Water quality index evaluation for major rivers in Gujarat. Environ Sci Pollut Res 28:1–9
Niyoyitungiye L, Giri A, Mishra B (2019) Assessment of physico-chemical characteristics of water at selected stations of Lake Tanganyika, Africa with special emphasis on pisciculture purposes. Int J Basic Appl Biol 6(3):211–217
Noori R, Berndtsson R, Hosseinzadeh M, Adamowski JF, Abyaneh MR (2019) A critical review of the application of the national sanitation foundation water quality index. Environ Pollut 244:575–587
Ongom R, Andama M, Lukubye B (2017) Physico-chemical quality of Lake Kyoga at selected landing sites and anthropogenic activities. J Water Resour Prot 9(11):1225–1243
OpiyoGetabuSitokiShitandiOgendi SAMLMAGM (2019) Application of the Carlson’s trophic state index for the assessment of trophic status of Lake Simbi ecosystem, a deep Alkaline-saline Lake in Kenya. Int J Fish Aquat Stud 7(4):327–333
Ozguven A, Demir Yetis A (2020) “Assessment of spatiotemporal water quality variations, impact analysis and trophic status of big Soda Lake Van, Turkey. Water Air Soil Pollut 231(6):260
Saturday A, Lyimo TJ, Machiwa J, Pamba S (2021) Spatio-temporal variations in physicochemical water quality parameters of Lake Bunyonyi. South Western Uganda SN Appl Sci 3(7):1–14
Saturday A, Lyimo TJ, Machiwa J, Pamba S (2022) Spatial and temporal variations of phytoplankton composition and biomass in Lake Bunyonyi. South Western Uganda Environ Monit Assess 194(4):1–17
Song J, Hou C, Liu Q, Wu X, Wang Y, Yi Y (2020) Spatial and temporal variations in the plankton community because of water and sediment regulation in the lower reaches of the Yellow river. J Clean Prod 261:120972. https://doi.org/10.1016/j.jclepro.2020.120972
Tibebe D, Kassa Y, Melaku A, Lakew S (2019) Investigation of spatio-temporal variations of selected water quality parameters and trophic status of Lake Tana for sustainable management, Ethiopia. Microchem J 148:374–384
Tibihika PDM, Okello W, Barekye A, Mbabazi D, Omony J, Kiggundu V (2016) Status of Kigezi minor lakes: a limnological survey in the lakes of Kisoro, Kabale and Rukungiri districts. Int J Water Resour and Environ Eng 8(5):60–73
Tiémoko GJ-L, Ouattara NK, Kouamé C-KY, Ouattara A, Gourène G (2020) Spatial and Temporal variation of faecal indicator bacteria in three reservoirs of ivory coast (Taabo, Kossou and Fae). J Environ Sci 6(1):408–411
Tilahun G, Ahlgren G (2010) Seasonal variations in phytoplankton biomass and primary production in the Ethiopian rift valley Lakes Ziway, Awassa and Chamo-the basis for fish production. Limnologica 40(4):330–342
Umer A, Assefa B, Fito J (2020) Spatial and seasonal variation of lake water quality: Beseka in the rift valley of Oromia region, Ethiopia. Int J Energ Water Resour 4(1):47–54
UNBS (2014) Uganda standards template—world trade organization (1st ed), Government of Uganda
WHO (2011) Guidelines for drinking water quality. WHO Chron 4(38):104–108
WHO (2008) Guidelines for drinking-water quality: incorporating first and second addenda to the third edition, vol 1, Recommendations. Geneva: WHO Press
WHO (2017) Guidelines for drinking-water quality: first addendum to the fourth edition
WHO (2018) A global overview of national regulations and standards for drinking-water quality. World Health Organization
Yu Y, Song X, Zhang Y, Zheng F (2020) Assessment of water quality using chemometrics and multivariate statistics: a case study in Chaobai river replenished by reclaimed water. North China Water 12(9):2551
Zhou L, Wang X, Zhang X, Zhao Y, Zhu P, Zhao X, Li X (2020) Spatiotemporal variations in nitrogen and phosphorus in a large man-made Lake and their relationships with human activities. Water 12(4):1106
Acknowledgements
The author would like to thank the management of NWSC Kampala for providing laboratory facilities.
Funding
The study was supported by the Directorate of Research and Publications of Kabale University, Uganda.
Author information
Authors and Affiliations
Contributions
AS conceived and designed the study, collected and analyzed data, and drafted the manuscript. SK assisted in data collection, analysis and revision of the manuscript for intellectual content. WB assisted in data collection, analysis and revision of the manuscript for intellectual content. All the authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saturday, A., Kangume, S. & Bamwerinde, W. Content and dynamics of nutrients in the surface water of shallow Lake Mulehe in Kisoro District, South–western Uganda. Appl Water Sci 13, 150 (2023). https://doi.org/10.1007/s13201-023-01953-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01953-y