Abstract
This study reports the significance of freshwater springs primarily in meeting drinking water demands besides offering various ecosystem services. We analyzed a total of eighteen hydrochemical quality parameters using standard methods from various representative springs of Anantnag district, Kashmir Himalaya. Groundwater quality profiles were generated in a GIS environment for each parameter. Additionally, statistical methods were employed to understand the interdependence of water quality parameters. Highly variable dissolved oxygen (0.4–9.2 mg L−1) and relatively higher values of nitrate ranging from 57 to 2668 µg L−1 noticed during the study may be mostly related to contamination from agricultural waste. The findings of this study revealed that the springs are predominantly hard water type as the water samples found were calcium-rich and exhibited higher total phosphorus in a few samples owing to limestone lithology in the catchment. Principal Component Analysis (PCA) to the data generated chiefly three components (VF1, VF2, and VF3) having Eigen values of 2.0 or more (2.28–5.37) contributing for 31.63%, 17.99% and 13.44% of the total variance, respectively. The water quality index (WQI) of the samples for drinking purpose ranged from good to excellent. In light of our findings, it is argued that springs offer a potential, although partial, solution to the drinking water demands of a burgeoning population in Indian Himalayan region. However, equally important is to have a thorough investigation of springs to explore the impacts of other forms of pollution, including heavy metals, pesticides and antibiotic wastes, which can diminish much-needed ecosystem services.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water quality issues are a major challenge that humanity is facing in the twenty-first century (UNESCO,UN Water, 2020). Improvements in water resources management and access to water supply and sanitation services is therefore becoming a challenge globally, which underpins the vision that humanity should enjoy uninterrupted, various goods and services that are related with water (WWAP 2019).Water security which has emerged as a prominent challenge (Srinavasan et al. 2017) has initiated dialogue on improving water resource security for the future because of projected water shortages due to climate change (Huntington 2006) and rising water demand across the globe has increased (Burek et al. 2016). Two-thirds of the human population is subjected to severe water scarcity, with 50% of the populations of China and India facing water shortage at least once a year, and 50 million people globally continually short of drinking water (Mekonnen and Hoekstra 2016; Yomo et al. 2019). Presently, maintaining the availability and safeguarding the quality of the freshwater resources on the global horizon are the most pressing of many environmental challenges (Bhat et al. 2022). Goldscheider et al. (2020) report that 15.2% of the global land surface is covered by karstic terrains and freshwater springs are known to supply partially or entirely 25–50% of drinking water to the world’s population (Ford and Williams 2007; Hartmann et al. 2014). United Nations Sustainable Development Goal (SDG) 6 is to ensure access to safe drinking water and sanitation to all by 2030 (United Nations 2018), and proper water and sanitation are critical to achieving SDGs of good health and gender equality. Springs are widely distributed across landscapes and sustain and support millions of people across globe including Indian Himalayan Region (Tambe et al. 2011; Risko 2018; Bhat and Pandit 2018, 2020; Bhat et al. 2020; Lone et al. 2020; Stevens et al. 2021).
India is the largest user of groundwater in the world, consuming an estimated 230 km3 of groundwater annually, over a quarter of the global total (Chatterjee and Purohit 2009). The quality of groundwater has deteriorated owing to the expansion of agriculture, industries and unplanned urbanization leading to severe stress on groundwater resources (Maréchal et al. 2018; Pande et al. 2020). Water quality is a prerequisite for sustainable water and sanitation in SDG 6, and is also equally important for many other SDGs related to health, food security and biodiversity. Growing population, urbanization and change in lifestyle have resulted in change in sewage quality characteristics as well as increase in volume of sewage, thereby potentially triggering nutrient and biological disruption of aquatic ecosystems (Gupta et al. 2018). Therefore, contamination of water resources is an emerging environmental problem worldwide, and water resources such as the springs of Kashmir, have sustained tremendous anthropogenic impacts from contamination, particularly nutrient enrichment (Lone et al. 2020), catchment scale land use changes (Rather et al. 2016), urbanization (Rashid and Aneaus 2019; Dar et al. 2021a), entry of untreated sewage (Parvaiz and Bhat 2014; Dar et al. 2020a; Qayoom et al. 2021; Bhat et al. 2020; Lone et al. 2020), eutrophication (Romshoo and Muslim 2011; Dar et al. 2020b), heavy metal (Ahmad 2014; Ahmad et al. 2016; Showqi et al. 2018; Qayoom et al. 2021), sediment load (Rashid and Aneaus 2019) which deteriorates their water quality (Vass 1980; Najar et al. 2014; Bhat and Pandit 2014; Dar et al. 2021b) along with adverse impact on aquatic life (Khan et al. 2004; Zutshi and Gopal 2000).
The karstic terrain of the Kashmir Valley is recognized for its many spring-fed streams (Coward et al. 1972). The water from its springs has created aquatic habitats with physico-chemical conditions that contrast greatly to surface fed streams and ponds (CGWB 2013). These unique habitats are usually characterized by constant temperatures, stable discharge regimes, sometimes low levels of dissolved oxygen and elevated but stable levels of ionic enrichment (Bhat and Pandit 2018). The springs in the valley are of remarkable importance and have been frequently modified for recreational and drinking water purposes with associated impacts on aquatic organisms. The spring habitats in the valley have a unique and huge potential for fish (trout) culture, which contributes approximately Rs. 300 lakhs annually to the State economy. Besides that, they provide drinking water in areas with rising burdens of population and pollution, irrigation of fields, especially during drought-ridden periods over the past two decades. Aridity increases the attractiveness of springs for washing, bathing, swimming, and other activities. Some hydro-geochemical research has been conducted on the groundwaters and springs of the Kashmir Valley (Bhat et al. 2010; Bhat and Pandit 2010a, b; Jehangir et al. 2011; Jeelani 2005, 2011, 2014; Hameed et al. 2018) and also in other parts of India (Saxena and Mondal 2003). These studies indicate that congruent carbonate dissolution (calcite dissolution, dedolomitization) and incongruent silicate weathering are the dominant processes controlling the groundwater quality in the south eastern part of the Kashmir Valley. Along Jhelum River in the southern part of Kashmir, the groundwater is controlled by lithology. Despite the role of springs across the world in urban water supply (Petric 2010), little attention has been devoted to research on the economics of spring’s service values (Nabhan 2008; Phillips et al. 2009).
Over the past 20 years, the Kashmir Valley has seen a major shift from agriculture to horticulture related to higher economic returns that, in turn, have resulted in massive increases in the use of fertilizers and pesticides. These compounds also add heavy metals into the aquatic system of the entire Jhelum River basin that flows through the Kashmir Valley. Keeping in mind the importance of spring waters, we conducted an investigation on the physico-chemical characteristics of spring waters in Anantnag district. That groundwater retention in karstic aquifers, such as those underlying the Kashmir Valley, can be very brief making surface contamination quickly transported and exported from the aquifer (Coward et al. 1972; Bhat and Pandit 2018). Thus, the study of pollution in such settings is important for understanding potential impacts to drinking water quality. Development impacts appear to be widespread throughout India, and we hypothesized that changing environmental conditions associated with increased development has increased impacts on Kashmir Valley springs, thereby reducing their sustainability. We sought to determine whether the drinking water quality of springs had deteriorated in relation to other ecosystem goods and services, such as washing, bathing, irrigation, livestock water, and pisciculture. This paper also may offer the policy inputs framing water security plan at District level envisaged under flagship effort of the Government of India’s National rural drinking water programme (i.e. movement towards ensuring drinking water security) in rural India. We are of this view that this study is in tandem with SDGS (Sustainable developmental goals) as well as national missions of India like Clean and Healthy India. We include a description of the ecosystem services that springs provide, and report on imminent challenges and threats of various types of pollutants which can undermine the quality and potential of spring use for ecosystem service providers. The majority of research on Kashmir springs has focused on hydrogeology, while that on ecosystem services and crenobiodiversity is sparse (Lone et al. 2021). In this article, apart from the water quality perspective through physicochemical parameters, we emphasize that an array of water pollution threats are looming over the future of the Kashmir Valley, affecting groundwater, springs, and surface waters. These pollution impacts merit attention and appropriate mitigation to ensure the sustainability of safe drinking water from springs in the region (Bhat et al. 2021). One of the many challenges facing springs in the Kashmir and Indian Himalayan Region is the lack of adequate information for management, as the studies conducted thus far have an academic orientation and were conducted on a small number of springs and for a particular area or district and for a shorter period of time. Such research therefore lacks the required direction and potential to offer clear policy guidance or data the management authorities are looking for at a bigger scale.
Material and methods
Study area
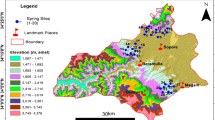
Kashmir valley harbours hundreds of springs that attract the tourists and thereby play an essential role in improving the local economy by providing visitors with an array of services. ‘Anantnag’ literally means ‘innumerable springs’ (Anant means countless and nag means springs in Kashmiri language). It is the southernmost district of Kashmir province lying between 75°03′30"–75°19′29"E longitude and 33°31′07"–33°54′30"N latitude, and covering an area of about 402 km2 (Fig. 1). The population of Kashmir valley is about 10,70,114 individuals as per 2011 census. The elevation of the study area varies from 1591 to 2708 m above sea level (m a.s.l). The area receives an average annual precipitation of 1240 mm/yr and has a temperate climate (Rashid et al. 2017). The surficial geology of the valley’s lower plains are dominated by unconsolidated Quaternary alluvium, while Triassic and Permo-Carboniferous strata and the Panjal volcanic field dominate the central eastern and northern regions, respectively (Fig. 1). Bore and tube wells yields of ~ 10–20 Ls−1 are sufficient in Triassic strata, but are low to moderate (~ 5–10 Ls−1) from hand dug and pumped wells in the northern Permo-Carboniferous formations.
Sampling and analysis
We selected thirty representative springs in this study of the Anantnag District for detailed geochemical analysis (Table 1, Fig. 2). We collected water samples in 1 L clean plastic bottles. Before the collection of samples, bottles were carefully washed with distilled water. Each sample was analysed for 18 different physicochemical parameters such as temperature, pH, conductivity, chloride, alkalinity, total hardness, calcium, ammonia, nitrite, nitrate, ortho-phosphorus, total phosphorus, iron, and sulphate (APHA 2005). The temperature of air and water was recorded by using a graduated Celsius thermometer. Electrical conductivity, pH, salinity and total dissolved solids were determined by using a Digital PCS Tester 35 multi-parameter probe. Dissolved oxygen was estimated by Winkler’s titration method. Parameters like free CO2 and alkalinity were determined by titrimetric methods. Chlorine was estimated by the argentometric method. Total hardness and calcium were measured by a complexometric method. Nitrogen, phosphorus, sulphate, and iron were analysed by spectrophotometric methods. Analysed parameters were interpreted with the cross-plots, their spatial distribution, cluster analysis and principal component analysis (PCA).
A water quality index (WQI) value (Gebrehiwot et al. 2011) was calculated for the thirty selected Anantnag springs, using eight hydro-chemical parameters. WQI values were classified into five types as “excellent water” to “unsuitable for drinking water”. During our survey discussions and interactions with users and relevant stakeholders informed us about the services provided by these springs in the study area.
Results and discussion
Descriptive analysis
Safeguarding groundwater quality from an ever-increasing enrichment and diversity of environmental contaminants is a large challenge (Damania et al. 2019). Reports regarding the rise in groundwater temperature and the widening of seasonal thermal variations in response to atmospheric temperature changes, besides their roles in ecosystem acknowledged in the research community (Kurylyk 2014; De Stasio et al. 2009). From the water temperature data records, all the 30 springs were found to be cold water springs having temperatures lower than the ambient air temperature. A perusal of the results indicated related to range of temperature in the studies springs from 9.5 to 20.0 °C (Fig. 3a). Only springs 5–7, 15, 17, 21, and 25 were warm springs (temperature range 16–20 °C), indicating that the groundwater had travelled through longer circulation paths having some hydrothermal activity (Nathenson et al. 2003). Some recent reports on more than 300 springs have shown the temperature range of spring waters between 18 and 22 °C. In one of the study on spring water temperatures authors have reported an increase of approximately 2 °C in the period from 2002 to 2017 (Kurylyk 2014). The maximum temperature (20 °C) was observed at Baleyar village (S-7). Water was found slightly acidic to alkaline (pH range: 6.8–8.3), with an average value of 7.4. The pH of most springs is basic in nature, which can be attributed to limestone-rich lithology of Kashmir valley (Al-Jiburi and Al-Basrawi 2013; Barakat et al. 2018). The value of dissolved oxygen (DO) concentration varied from 0.4 to 9.2 mg L−1, with an average of 3.7 mg L−1 (Fig. 3b). Variation in the concentration of DO is due to differences in water temperature (Singh et al. 2014), as DO concentration is inversely related to water temperature. A similar trend was observed in all springs, with S-5 showing the lowest DO level (0.5 mg L−1) at the temperature of 18 °C. Besides high temperature, another cause for such low levels of DO can be ascribed to the relatively higher amount of sulphur present in the spring under question (Bhat 2015).The highest values of DO were observed at S-1 (Kokernag), S2 (Verinag) and S3 (Achabal) which had low temperature. The presence of relatively high oxygen at these sites seems to be a function of oxygen producing periphytic algal population found very much visible during the survey in these springs. It is important to mention here that the dissolved oxygen is normally not a primary water quality parameter considered by regulatory agencies, but is negatively related to BOD and COD, and therefore offer insight into the status of water quality. At the same time, DO plays a significant role in aesthetics and ecosystem functioning (Chen et al. 2019). In our study about 77% of the springs recorded DO levels less than 5 mg L−1, however this does not affect the potability of water of these springs, as it normally does not have any harmful impacts on the human system. The springs, in general, were rich in CO2 (Fig. 3b) because of the dissolution of carbonates from the catchment in addition to inputs from bacterial respiration and organic matter decomposition (Maas and Wicks 2017). Electrical conductivity (EC) was found to lie in the range of 168–855 µScm−1, indicating low to moderate mineral content (Fig. 3c), with contamination by inorganic fertilizers and domestic sewage potentially responsible for higher levels of conductivity (> 700 µS cm−1) (Kumar et al. 1996) found in the springs like 13, 17, 19, 27 and 28. The overall higher conductivity of springs is also likely attributed to the high residence time of water in the aquifer, which prolongs its interaction with host rock (Jeelani 2010). Broadly, springs having water temperature from 9.5–12 like 1, 2 and 3 were usually found to have the lower EC values, with the notable exception of springs like 21, 25 and 26. As EC and TDS are strongly correlated (Abdul Wahid 2013), the values of TDS were found in proportion with EC. The concentration of salinity is directly related to the aquifer geology and its chemical characteristics (Al-Naeem 2015).
Cross-plots between various water quality parameters of the spring waters at different study sites a Water temperature (°C) and pH b CO2 (mg L−1) and DO (mg L−1) c TDS (mg L−1) and Conductivity (µs cm−1) d Sulphate (mg L−1) and Chloride (mg L−1) e Total hardness (mg L−1) and Calcium content (mg L−1) f Total phosphorus (µg L−1) and Orthophosphorous (µg L−1) g Nitrate (µg L−1) and Ammonia(µg L−1)
The anions of the spring waters revealed the dominance of Cl− ions over other ions with usual ionic progression as Cl− > SO42−. The Cl−and SO42− values ranged from 5–40 mg L−1 and 1–33 mg L−1, respectively, in the study area (Fig. 3d). Differences in SO42− values may be due to prevailing land-use type and the extent of impregnation of the aquifer bedrock with CaSO4 (Cole 1983). Relatively low concentration of chloride can indicate negligible impact on springs, as compared to studies conducted elsewhere reporting the increased concentration due entry of sewage directly or indirectly in the springs (Paramsivam and Srinavasan 1981; Bhat and Pandit 2018). Higher concentrations of alkalinity at some springs are chiefly contributed by the presence of bicarbonates (Reda 2015). The permissible limit for drinking water of alkalinity and chloride prescribed by WHO (2011) is given as 200 mg L−1 and 250 mg L−1, respectively (Table 2).
Hardness is mainly determined by the presence of Ca2+ and Mg2+ ions in water. Hardness is not a pollution indicator parameter: it indicates only water quality. The total hardness values ranged from 72 to 340 mg L−1 with an overall mean of 193 mg L−1, indicating the water in all the springs is hard in nature (Fig. 3e). The concentrations of cations of Ca2+ and Mg2+ ranged from 36–289 mg L−1 and 19–112 mg L−1, respectively. Ca2+ and Mg2+ owe their origins to lacustrine deposits as sedimentary rocks like limestone, gypsum, and dolomite. The dominance of calcium ions over magnesium ions is attributable to abundance of carbonate strata in the study area. It was observed that the Ca2+ and Mg2+ are correlated with hardness with the correlation coefficients of 0.96 and 0.46, respectively. The desirable limit for hardness is 300 mg L−1 (WHO 2011) but for the Ca2+ and Mg2+the desired limits is 75 mg L−1 and 30 mg L−1, respectively (Table 2). The majority of the springs studied fall into hard and very hard water categories.
The concentration of iron varied from 1–764 µg L−1 with an overall mean of 76 µg L−1, well below the permissible limit (1000 µg L−1) of drinking water (WHO 2011). The maximum value of 764 µg L−1 was obtained at S-3 (Achabal). In addition to enrichment by organic wastes, the source of iron (Fe) may be due to the presence of iron rich minerals in the catchment of these springs (Rao 2007). During the investigation, we could see the concentrations of orthophosphorus and total phosphorus fluctuated from 7–401 and 44–2837 µg L−1, respectively (Fig. 3f). Source of phosphates can be artificial or anthropogenic, depending on human activities in the area. The slightly higher concentration of phosphates in some springs provides clear evidence of the direct influence of agricultural and horticultural activities being undertaken in the catchment of these springs where there is heavy use of phosphate fertilizers (Kipngetich et al. 2013). Since, the Jhelum basin receives phosphate loading through agriculture and horticulture uses, which sustain the livelihood of the major portion of the population in Kashmir. This pollution jeopardizes the ecosystem services of springs on a long-term basis (Charlton et al. 2018). Further, rocks in the whole Panjal traps are calcium-rich in combination with phosphate, which on dissolution releases exceedingly high concentrations of phosphorus in spring waters in the area. Almost 20% of the springs located in the northwestern portion of the District displayed higher concentrations of orthophosphorus and total phosphorus, whereas the rest of the 80% springs showed the concentrations which have been reported by few earlier works as well (Jeelani et al. 2001, 2014; Bhat and Pandit 2018; Bhat et al. 2021).
Nitrogen in the form of nitrate (NO3−), nitrite (NO2−), and ammonia (NH3+) present in natural water evoke great interest because of their nutrient values, thereby being limiting factors for many bio-chemical processes. Nitrate concentration in the present study varied from 57 µg L−1 at S-5 to 2668 µg L−1 at S-19 (Fig. 3g). A higher concentration of nitrate in groundwater is due to the excessive use of fertilizers and the addition of animal and human waste (Mondal et al. 2008; Bhat and Pandit 2010a, b). Lower nitrate concentrations in some springs are due to minimum agriculture activities in the immediate catchment. Decomposition of organic matter is also reported as an important source of nitrate in the springs. However, there is emerging evidence, that the threshold of 10 mg L−1 may be too high, as the nitrate levels of 3.87 mg L−1 have been reported to be associated with colorectal cancer (Schullehner et al. 2018).
The nitrite values of springs were recorded in the range of 1–495 µg L−1 with an average of 30 µg L−1. The higher variation in the nitrite–nitrogen in groundwater may be due to the leaching of domestic sewage through soil stratum, as reported by Sheikh et al. (2013). Ammonia values in most of sites were recorded in a range of 2–16 µg L−1 (Fig. 3g). It is more or less negatively correlated with nitrate concentrations of spring water samples with correlation coefficient of − 0.26. In general, agricultural sources seem to be the main source of nitrogen in these springs, as well as decomposition of organic matter and domestic sewage.
Spatial distribution of the selective hydro chemical constituents
We prepared spatial distribution maps of the selective hydrochemical parameters of the spring waters to assess its appropriateness for drinking water practices using kringing technique. TDS values varied from 120 to 608 mg L−1 with a mean of 315 mg L−1, which are less than the desirable limit for drinking water use (Fig. 4a). It was observed more than exceeding the permissible limit (> 500 mg L−1, WHO 2011) at the Springs S-13, 19, 27 and 28, which are located in central-eastern and western parts. The DO varied from 0.4–9.2 mg L−1, although it has been noted that > 5.0 mg L−1 of DO (in about 33% springs) have better impacts on aquatic ecosystem health. It had been found in half the portion of the study are located in the south and also at the springs S-11, 18 and 24 in the northern part (Fig. 4b). The spatial distribution map of Fe content had been prepared as is shown in Fig. 4c. Values of iron varied from 1 to 764 µg L−1. Here, we could find that except for Achabal Spring all values were within desirable and permissible limits of drinking water quality. It was only here Achabal spring wherein concentration was exceeding the desirable limit of 300 µg L−1 but still well within the persimible limit of 1000 µg L−1 (Table 2). On the other hand, we observed total phosphorus concentration in the range of 44–2837 µg L−1 and its spatial distribution depicted in Fig. 4d. It shows that a comparatively higher value were encountered in the N-W of the area, where rocks are calcium-rich within Panjal traps which bound phosphate.
Statistical analysis
Cluster analysis and principal component analysis (PCA)/factor analysis
The cluster analysis is a useful method for finding homogeneity groups within data from chemical spring water analysis. Spring waters were grouped into three distinct classes by cluster analysis (Fig. 5). Cluster 1 corresponds to four sites (S-6, 10, 12 and 14), Cluster 2 contains eleven sites (S-1, 5, 7, 8, 11, 15, 18, 21, 26, 29 and 30), and Cluster 3 contains fifteen sites (S-2, 3, 4, 9, 13, 16, 17, 19, 22, 23, 24, 25, 27, 20 and 28). From cluster analysis, the variability of hydrochemistry was mainly related to rock-water interaction, dissolution of carbonates, and likely influences of anthropogenic activities (agricultural and horticultural) in the spring catchment area.
We carried out the PCA to extract the most important factor which showed similarity between the different sampling sites. This analysis identified a reduced number of 3 varifactors (i.e., VF1, VF2, and VF3), indicating that 63% of temporal and spatial changes in water quality of springs (Fig. 6). VF1 elucidated 31.63% of the total change with strong positive loading on parametres like water temperature, pH, electrical conductivity, CO2, alkalinity, chloride, TH, Ca2+ and Mg2+, nitrate, nitrite, orthophosphorous and total phosphorus (Fig. 7a). On the other hand, negative loads were detected for the DO, Fe and ammonia. VF2 explained about 17.99% of total variance of electrical conductance, CO2, DO, alkalinity, chloride, Mg2+, orthophosphorous and total phosphorus, Fe, SO42−, nitrate and ammonia (Fig. 7b). The VF3 described about 13.44% of the total variance had a strong positive loading only on the spring water temperature, CO2, orthophosphorous, total phosphorus, sulphate and nitrite (Fig. 7c), but others had negative loadings.
Factor scores for the a factor 1 b factor 2 c factor 3 of hydro-chemical constituents in the spring water samples. Abbreviations of the water parameters are: Water temperature (WT), pH, Electrical conductivity (EC), Salinity (Sal), Total dissolved solids (TDS), Dissolved oxygen (DO), Free carbon dioxide (CO2), Total alkalinity (Alk), Chloride (Chl), Total hardness (TH), Calcium hardness (CH), Magnesium hardness (MH), Sulphate (Sul), Iron Nitrate (Nit), Nitrite (Nitri), Ammonia (Am), Ortho-phosphorus (OP), and Total phosphorus (TP)
Water quality index (WQI), socioeconomic and governance aspects
A significant parameter, WQI is mainly adopted for assessing spring water quality and its fitness for drinking purposes (Gorde and Jadhav 2013). Weighted quality index method classifies water quality according to degrees of purity using the methods of Brown et al. (1972) and Ahmad (2014). Being an effective tool to examine the extent of water, it can be used well in the execution of water quality upgrading programmes. It is one of the collective indices that have been acknowledged as a rating that reflects the composite influence on the overall quality of information of specific water quality data (Das et al. 2013).
Values of WQI were calculated for the spring samples and the resultant index thus generated varied from 35 to 82 with an average of 56. As we know that WQI values are grouped into 5 types for drinking purpose in ranges of 0–50, 50–100, 100–200, 200–300 and > 300 for the excellent, very good, poor, very poor and unsuitable drinking waters, respectively. The analysis revealed that all the physico-chemical water parameters are well within the highest desirable limit or maximum permissible limit prescribed by the WHO (2011) (Table 3). From the analysis of the above results, it has been found that the overall water quality of 30 springs falls within the excellent (12 spring sites) to very good (18 spring sites) category of water quality class based on water quality index values which is suitable for both irrigation and drinking use (Fig. 8) as reported here and elsewhere (Bhat et al. 2020; Bhat and Pandit 2020; Lone et al. 2020). Given the type of water quality and other ecosystem services these springs provide (Fig. 9) and the corresponding threats operating at various levels and magnitudes (Bhat et al. 2021), it is prayed to have the in-depth investigation and monitoring programs are needed on the looming threats to protect and safeguard the quality and character of these spring ecosystems.
Conclusion
Hydrochemical signatures of spring have important roles in evaluation of drinking water quality. We could get the excellent water quality impression from our data purely based on physicho-chemical parametres being used through the Water Quality index. However, the concentration of phosphorus level raise some genuine concern regarding the potential to trigger eutrophication in spring ecosystems which has not been worked out in these systems till now. This may also impede the services of springs like economic, ecosystem and social services of the spring ecosystems. Therefore, we suggest that regular monitoring of selected springs is needed to facilitate rapid responses to various threats, like nutrient enrichment and pollution from heavy metals, pesticides and micro plastics. We also argue that the costs involved in treating the drinking water from other sources can be saved if springs are tapped, connected to water supply schemes, and managed properly. However, ecological integrity of spring ecosystems in this process should be protected and safeguarded.
Change history
24 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13201-022-01741-0
References
Abdul Wahid SJ (2013) Water quality index of Delizhiyan springs and shawrawariver within Soran district, Erbil, Kurdistan region of Iraq. J Appl Environ Biol Sci 3:40–48
Ahmad AB (2014) Evaluation of groundwater quality index for drinking purpose from some villages around Darbandikhan district, Kurdistan Region Iraq IOSR. J Agric Vet Sci 7(9):34–41
Ahmad SS, Reshi ZA, Shah MA, Rashid I, Ara R, Andrabi SM (2016) Heavy metal accumulation in the leaves of potamogeton natans and ceratophyllum demersum in a Himalayan Ramsar site: management implications. Wetl Ecol Manag 24(4):469–475
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC USA
Al-Jiburi HK, Al-Basrawi NH (2013) Hydrogeological map of Iraq, scale 1: 1000 000, 2013. Iraqi Bull Geol Min 11:17–26
Al-Naeem AA (2015) Monitoring of groundwater salinity for water resources management in irrigated areas of Al-Jouf region Saudi Arabia. Res J Environ Sci 9(6):256–269. https://doi.org/10.3923/rjes.2015.256.269
Barakat A, Meddah R, Afdali M, Touhami F (2018) Physicochemical and microbial assessment of spring water quality for drinking supply in piedmont of Béni-Mellal Atlas (Morocco). Phys Chem Earth Parts a/b/c 104:39–46
Bhat BB (2015) Physico chemical analysis of River Baldi Sahastradhara (Dehradun). Int J Sci Technoledge 3(8):292–299
Bhat SU, Pandit AK (2010a) Comparative ecology of some freshwater springs of Kashmir Himalaya. Ph.D. Thesis, University of Kashmir Srinagar
Bhat SU, Pandit AK (2018) Hydrochemical characteristics of some typical freshwater springs-a case study of Kashmir Valley springs. Int J Water Resour Arid Environ 7(1):90–100
Bhat SU, Pandit AK (2010b) Limnochemistry of three freshwater springs of Kashmir Himalaya. Hydro Nepal 7:54–58
Bhat SU, Dar SA, Hamid I (2022) A critical appraisal of the status and hydrogeochemical characteristics of freshwater springs in Kashmir Valley. Sci Rep 12:5817. https://doi.org/10.1038/s41598-022-09906-2
Bhat SU, Dar SA, Sabha I (2021) Assessment of threats to freshwater spring ecosystems. Imperiled: the encyclopedia of conservation. https://doi.org/10.1016/B978-0-12-821139-7.00068-4
Bhat SU, Mushtaq S, Qayoom U, Sabha I (2020) Water quality scenario of Kashmir Himalayan springs—a case study of Baramulla district Kashmir Valley. Water Air Soil Pollut 231:454
Bhat SU, Pandit AK (2020) Water quality assessment and monitoring of Kashmir Himalayan freshwater springs—a case study. J Aquat Ecosyst Health Manag 23:274–287
Bhat SU, Pandit AK, Mudathir R (2010) Limnological Investigation of three freshwater springs of pulwama district- Kashmir valley. Rec Res Sci Technol 2(2):88–94
Brown RM, McCleiland NJ, Deiniger RA, O’ Connor MFA (1972) Water quality index-crossing the physical barrier In: Jenkis SH (ed.), Proceedings in international conference on water pollution research jerusalem, 6, p 787–797
Burek P, Satoh Y, Fischer G, Kahil MT, Scherzer A, Tramberend S, Nava LF, Wada Y, Eisner S, Flörke M, Hanasaki N, Magnuszewski P, Cosgrove B, Wiberg D (2016) Water Futures and solution: fast track initiative (Final Report). IIASA Working Paper. International Institute for Applied Systems Analysis (IIASA), Laxenburg, Austria. https://pure.iiasa.ac.at/13008/
Central Groundwater Board (CGWB) (2013) Ground water information booklet of Chandel district, Manipur, Technical Report Series: D, No: 10/2013-14, Central Groundwater Board, North Eastern Region, Ministry of Water Resources
Charlton MB, Bowes MJ, Hutchins MG, Orr HG, Soley R, Davison P (2018) Mapping eutrophication risk from climate change: future phosphorus concentrations in english Rivers. Sci Total Environ 613:1510–1526. https://doi.org/10.1016/j.scitotenv.2017.07.218
Chatterjee R, Purohit RR (2009) Estimation of replenishable groundwater resources of India and their status of utilization. Curr Sci 96(12):1581–1591
Chen J, Wang Y, Li F, Liu Z (2019) Aquatic ecosystem health assessment of a typical sub-basin of the Liao River based on entropy weights and a fuzzy comprehensive evaluation method. Sci Rep 9:14045. https://doi.org/10.1038/s41598-019-50499-0
Cole DR, Ohmoto H, Lasaga AC (1983) Isotopic exchange in mineral-fluid systems. I. Theoretical evaluation of oxygen isotopic exchange accompanying surface reactions and diffusion. Geochimica et Cosmochimica Acta 47(10):1681–1693
Coward JMH, Waltham AC, Bowser RJ (1972) Karst springs in the Vale of Kashmir. J Hydrol 16(3):213–223
Dar SA, Bhat SU, Aneaus S, Rashid I (2020a) A geospatial approach for limnological characterization of Nigeen Lake Kashmir Himalaya. Environ Monit Assess 192:121. https://doi.org/10.1007/s10661-020-8091-y
Dar SA, Bhat SU, Rashid I, Dar SA (2020b) Current status of wetlands in Srinagar city: threats, management strategies, and future perspectives. Front Environ Sci 7:199
Damania R, Desbureaux S, Aude-Sophie Rodella AS, Jason RJ, Zaveri E (2019) Quality unknown: the invisible water crisis. World Bank, Washington, DC
Dar SA, Hamid A, Rashid I, Bhat SU (2021a) Identification of anthropogenic contribution to wetland degradation: insights from the environmetric techniques Stoch. Environ Res Risk Assess. https://doi.org/10.1007/s00477-021-02121-x
Das S, Roy PK, Mazumdar A (2013) Development of water quality index for groundwater in Kolkata city, West Bengal India. ARPN J Eng Appl Sci 8(12):1054–1058
Dar SA, Rashid I, Bhat SU (2021b) Linking land system changes (1980–2017) with the trophic status of an urban wetland: implications for wetland management. Environ Monit Assess 193:710. https://doi.org/10.1007/s10661-021-09476-2
De Stasio BT, Golemgeski T, Livingstone DM (2009) Temperature as a driving factor in aquatic ecosystems. Encyclo Inland Waters. https://doi.org/10.1016/B978-0-12-409548-9.09051-5
Ford D, Williams P (2007) Karst hydrogeology to managing, restoring and conserving springs in the geomorphology. Desert Research Institute, England, Western United States, p 562
Gebrehiwot AB, Tadesse N, Jigar E (2011) Application of water quality index to assess suitability of groundwater quality for drinking purposes in Hantebet watershed, tigray Northern Ethiopia. J Food Agri Sci 1(1):22–30
Gorde SP, Jadhav MV (2013) Assessment of water quality parameters: a review. Int J Eng Res Appl 3(6):2029–2035
Goldscheider N, Chen Z, Auler AS, Bakalowicz M, Broda S, Drew D, Hartmann J, Jiang G, Moosdorf N, Stevanović Z, Veni G (2020) Global distribution of carbonate rocks and karst water resources. Hydrogeol J 28:1661–1677. https://doi.org/10.1007/s10040-020-02139-5
Gupta M, Santoro DHD, Torfs E, Doucet J, Van Peter AA, Vanrolleghen NG (2018) Experimental assessment an validation of quantification method for cellulose content in muncipal waste water and sludge. Environ Sci Pollut Res 25(17):16743–16753
Hameed A, Bhat SU, Jindal T, Sabha I, Lone SH (2018) Water quality monitoring of some freshwater springs in hazratbal tehsil, Srinagar, Kashmir Himalaya. J Himal Ecol Sustain Dev 13:61–74
Hartmann AN, Goldscheider T, Wagener JL, Weiler M (2014) Karst water resources in a changing world: review of hydrological modeling approaches. Rev Geophys. https://doi.org/10.1002/2013RG000443
Huntington TG (2006) Evidence for intensification of the global water cycle: review and synthesis. J Hydrol 319(1–4):83–95
Jeelani G (2005) Chemical quality of the spring waters of Anantnag, Kashmir. J Geol Soc India 66:453–462
Jeelani G (2010) Chemical and microbial contamination of anantnag springs, Kashmir Valley. J Himal Ecol Sustain Dev 5:176–183
Jeelani G, Bhat NA, Shivanna K, Bhat MY (2011) Geochemical characterization of surface water and spring water in SE Kashmir Valley, western Himalaya: implications to water–rock interaction. J Earth Syst Sci 120(5):921–932
Jeelani GH, Shah RA, Hussain A (2014) Hydrogeochemical assessment of groundwater in Kashmir Valley India. J Earth Syst Sci 123(5):1031–1043
Jehangir A, Tanver A, Yousuf AR, Masood A, Naqash AH (2011) Geochemistry and irrigation quality of groundwater along river Jhelum in south Kashmir India. Recent Res Sci Technol 3(6):57–63
Khan FI, Husain T, Hejazi R (2004) An overview and analysis of site remediation technologies. J Environ Manag 71(2):95–122
Kipngetich TE, Hillary M, Swamy TA (2013) Determination of levels of phosphates and sulfates in domestic water from three selected springs in Nandi county Kenya. Int J Pharm Life Sci 4(7):2828–2833
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1996) Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol 29(5):1232–1238
Kurylyk BL, MacQuarrie KTB, Voss CL (2014) Climate change impacts on the temperature and magnitude of groundwater discharge from shallow unconfined aquifers. Water Resour Res 50:3253–3274
Lone SA, Bhat SU, Hamid A, Bhat FA, Kumar A (2020) Quality assessment of springs for drinking water in the Himalaya of South Kashmir India. Environ Sci Pollut Res 26(2):2279–2300
Lone SA, Hamid A, Bhat SU (2021) Algal community dynamics and underlying driving factors in some crenic habitats of Kashmir Himalaya. Water Air Soil Pollut 232(3):1–14
Maas BJ, Wicks CM (2017) CO2 Outgassing from spring waters. Aquat Geo Chem 23:53–60. https://doi.org/10.1007/s10498-016-9302-6
Maréchal JC, Selles A, Dewandel B, Boisson A, Perrin J, Ahmed S (2018) An observatory of groundwater in crystalline rock aquifers exposed to a changing environment: Hyderabad, India. Vadose Zone J 17:180076. https://doi.org/10.2136/vzj2018.04.0076
Mekonnen MM, Hoekstra AY (2016) Four billion people facing severe water scarcity. Sci Adv. https://doi.org/10.1126/sciadv.1500323
Mondal NC, Saxena VK, Singh VS (2008) Occurrence of elevated nitrate in groundwaters of Krishna delta India. Afr J Environ Sci Technol 2(9):265–271
Nabhan GP (2008) Plant diversity influenced by indigenous management of freshwater springs. In: Stevens LE, Meretsky VJ (eds) Flora of Quitovac, Sonora, Mexico. Aridland Springs in North America: Ecology and Conservation. University of Arizona Press, Tucson, pp 248–68
Najar IA, Khan AB, Hai A (2014) Changing climatic conditions and seasonal variability in surface water quality of a shallow valley lake, Kashmir, India. In: Proceeding of the international conference on Green India: strategic knowledge for compacting climate change: prospects and challenges, Excel India Publishers, p 87–97
Nathenson M, Thompson JM, White LD (2003) Slightly thermal springs and non-thermal springs at Mount Shasta, California: chemistry and recharge elevations. J Volcanol Geoth Res 121:137–153
Pande CB, Moharir KN, Singh SK (2020) Groundwater evaluation for drinking purposes using statistical index: study of Akola and Buldhana districts of Maharashtra, India. Environ Dev Sustain 22:7453–7471. https://doi.org/10.1007/s10668-019-00531-013
Paramsivam M, Srinivasan A (1981) Changes in algal flora due to pollution in Cavery River Indian. J Environ Helth 23(3):222–238
Parvaiz S, Bhat SU (2014) Searching for water quality improvement in Dal lake, Srinagar, Kashmir. J Himalayan Ecol Sustain Dev 9:51–64
Petric M (2010) Characterization, exploitation, and protection of the Malenšcica karst spring, slovenia. In: Kresic N, Stevanovic Z (eds) Groundwater hydrology of springs engineering, theory, management, and sustainability. Butterworth-Heinemann, Oxford, pp 428–441
Phillips DA Jr, Van Pool CS, Van Pool TL (2009) The horned serpent tradition in the North American Southwest. In: VanPool C, VanPool TL, Phillips DA (eds) Religion in the prehispanic Southwest. Altamira Press, Lanham, pp 17–30
Qayoom U, Bhat SU, Ahmad I, Kumar A (2021) Assessment of potential risks of heavy metals from wastewater treatment plants of Srinagar city, Kashmir. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03612-8
Rao NS (2007) Distribution of iron in the surface and groundwaters of East Godavari district, Andhra Pradesh, India. Environ Geol 52(8):1455–1465
Rashid I, Romshoo SA, Abdullah T (2017) The recent deglaciation of Kolahoi valley in Kashmir Himalaya, India in response to the changing climate. J Asian Earth Sci 138:38-50
Rashid I, Aneaus S (2019) High-resolution earth observation data for assessing the impact of land system changes on wetland health in Kashmir Himalaya India. Arab J Geosci 12(15):1–13
Rather MI, Rashid I, Shahi N, Murtaza KO, Hassan K, Yousuf AR, Romshoo SA, Shah IY (2016) Massive land system changes impact water quality of the Jhelum River in Kashmir Himalaya. Environ Monitor Assess 188(3):1–20
Reda AH (2015) Assessment of physico chemical quality of spring water in Arbamich Ethopia. J Environ Anal Chem 2(5):2380–2391
Risko J (2018) Sacred springs: perceptions of religion and water in village communities of Uttarakhand. Independent study project (ISP) collection 2852. https://digitalcollections.sit.edu/isp_collection/2852
Romshoo SA, Muslim M (2011) Geospatial modeling for assessing the nutrient load of a Himalayan lake. Environ Earth Sci 64(5):1269–1282
Saxena VK, Mondal NC (2003) Application of water chemistry for the estimation of aquifer temperature puttur hot spring, Karnataka India. J Appl Hydrol 14(3):17–21
Showqi I, Lone FA, Naikoo M (2018) Preliminary assessment of heavy metals in water, sediment and macrophyte (Lemna minor) collected from Anchar Lake Kashmir India. Appl Water Sci 8(3):1–11
Schullehner J, Hansen B, Thygesen M, Pedersen CB, Sigsgaard T (2018) Nitrate in drinking water and colorectal cancer risk: a nationwide population-based cohort study. Int J Cancer 143(1):73–79. https://doi.org/10.1002/ijc.31306
Sheikh MA, Dar IY, Yaseen S, Pal A, Pandit AK (2013) A study of physico-chemical characteristics of three fresh water springs of Kashmir Himalaya India. Int J Water Res Environ Eng 5(6):328–331
Singh S, Negi RS, Dhanai R (2014) A study of physico-chemical parameters of springs around Srinagar Garhwal valley Uttarakhand. Int J Eng Dev Res 2(4):3885–3886
Srinivasan V, Konar M, Sivapalan M (2017) A dynamic framework for water security. Water Secur 1:12–20
Stevens LE, Ally AA, Voldoire O (2021) The ecological integrity of spring ecosystems: a global review. Imperiled: the encyclopedia of conservation, Elsevier. https://doi.org/10.1016/B978-0-12-82119-7.00111-2.
Tambe S, Kharel G, Arrawatia ML, Kulkarni H, Mahamuni K, Ganeriwala AK (2011) Reviving dying springs: climate change adaptation experiments from the Sikkim Himalaya. Mt Res Dev 32(1):62–72
United Nations-UN (2018) European global navigation satellite system and copernicus: supporting the sustainable development goals: building blocks towards the 2030 agenda. United Nations Office, Vienna
UNESCO, UN-Water, (2020) United Nations World Water Development Report 2020: Water and Climate Change, Paris, UNESCO
Vass KK (1980) On the trophic status and conservation of Kashmir lakes. Hydrobiologia 68(1):9–15
World Health Organization (WHO) (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Washington DC, p 541
WWAP (UNESCO World Water Assessment Programme) (2019) The united nations world water development report 2019: leaving no one behind. UNESCO, Paris
Yomo M, Mourad KA, Gnazou MDT (2019) Examining water security in the challenging environment in togo, West Africa. Water 11:231. https://doi.org/10.3390/w11020231
Zutshi DP, Gopal B (2000) Himalayan lake ecosystems: current issues and threat perceptions. Intern Ver Für Theor Und Angew Limnol Verh 27(4):2167–2170
Acknowledgements
The authors would like to express their sincere appreciation to Head, Department of Environmental Science, University of Kashmir for all kind of facilities provided in order to complete this study. Thanks to the two anonymous reviewers whose detailed and useful comments improved the manuscript.
Funding
The authors acknowledge the financial support of Department of Environmental Science, University of Kashmir for carrying out this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to updating of figures 8 and 9
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhat, S.U., Nisa, A.U., Sabha, I. et al. Spring water quality assessment of Anantnag district of Kashmir Himalaya: towards understanding the looming threats to spring ecosystem services. Appl Water Sci 12, 180 (2022). https://doi.org/10.1007/s13201-022-01691-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01691-7