Abstract
Water is a vital and widely spread component required for sustaining the life. Groundwater is vastly valuable source and it is extremely beneficial for the human beings. But, the toxic elements release from sources such as industries, landfills as well as non-point causes of pollution such as pesticides and fertilizer from the past year showed high levels of pollution in ground water, hence this is very crucial for evaluating the water quality not only for it’s existing usage, but also a its capacity to develop as a sustainable source of water for human utilization. In rural areas of India, Groundwater is a significant water-drinking source. In Bhokardan area of Jalna District, quality of ground water is great significance, because it is a key alternative source of domestic supply along with drinking water and peoples residing there facing several water quality issues for drinking purpose. In this current research work, an endeavor has been developed to investigate the quality of groundwater as well as spatial distribution by utilizing Remote Sensing along with GIS approaches. Water quality analysis was performed for developing the quality index of water, by utilizing 12 quality parameters of water like Alkalinity, pH, Electrical Conductivity, Fluoride, Chloride, Nitrate, Sulphate, Potassium, Total Hardness, E. coli, Turbidity and Total Dissolved Solids measured at 35 different selected locations in this research work. Spatial distribution map showed that each region of research area falls under category of “Poor water” and “Very poor water” except some sites at Northern regions of the area which falls under “Good water” category during pre-monsoon, whereas most of the sites from Northern and Southern part of the region shifted category from poor to good water along with very poor to poor water during post-monsoon season. The quality index data of water of the current research disclosed that, the high WQI “Water Quality Index” values in the samples of groundwater were principally due to the occurrence of higher values of turbidity and E. Coli. The higher level of water quality parameters like TH, EC, alkalinity, potassium, TDS, chloride and fluoride were also accountable for high values of WQI in this research work. None of the location falls under excellent quality for water during pre-monsoon as well as post-monsoon season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water quality and water supply have become a significant global problem as a consequence of enhanced population growth in industrial along with agriculture development and existing climate change contributes to shifts in the pattern of precipitation (Awodumi and Akeasa 2017). Groundwater is among the most critical natural and abundant resources and a main water supply source worldwide. Water quality is a critical problem for human beings as it is closely related to human wellbeing (P. Balakrishnan 2012). Groundwater is important not only because of its sustainability but because of its consistently high efficiency (Abou Zakhem and Hafez 2015). Various anthropogenic along with natural behaviors such as irrigation methods and practices, geology and regional climate are decreasing the quality of water. Rapid growth in industrialization and urbanization contributes to a degradation of the standard of surface as well as ground water (M.S.S. & Reddy, Chinna & Rao, R.S. Venkateshwar, Piska,(Srinivas et al. 2000).
Ground water quality is reliant on sub-surface geochemical procedures, anthropogenic activities, local surface water, precipitation and recharged water quality. Sequential variations in the composition as well as origin of recharged water, human along with hydrologic factors are the major cause of changes in quality of groundwater. Quality of water is affected by Water pollution as well as hygiene, economic development and social capital, risks to human and other live species (Milovanovic 2007; Reza and Singh 2010). While groundwater supplies have significant part to play in fulfilling water demand in arid- as well as semi-arid areas, many of them are being overtaken by over-use (Hosseini Moghari et al. 2015). Continuous and satisfactory supplies of drinking water from ground aquifer are significant for sustained agriculture, industry and domestic use all over large semi-arid areas of India (Murthy 2000). Ground water is major source of water for the past two decades due to growing demand of water, which leads to water shortage. The level of ground water is decreasing gradually, due to over exploitation therefore this is important to begin investigations concerned with ground water qualification and quantification that are the main criteria to plan for its management and utilization (Pandian and Jeyachandran 2014). The issues of ground water are the big concerns in the all over country. The control of developments in essential wetlands, in particular for arid and semi-arid environments in India, includes both salinization as well as overuse of agrochemicals. Principally, monitoring of concentration and trends of Fluoride and Nitrates in the aquifers for agriculture, industrialized and wholly polluted areas are essential and criteria for contamination must be improved (R.M.Bhardwaj 2005).
Biological, chemical as well as physical properties of water are well defined the water quality. Until its usage for different applications, for example industrial, recreational, agricultural, and drinking is essential (Khan et al. 2003). WQIs “Water Quality Indexes” are the usually utilized ways of measuring the quality state of water. Nevertheless, other limitations, such as a lack of appropriate resources or a lack of decision-making complexity, restrict such indexes (Hosseini Moghari et al. 2015). WQI in the United States has been primarily defined by Horton, (1965) by taking into consideration the ten most widely used metrics of water quality such as pH, and dissolved oxygen. The method is commonly employed and accepted in Asia, Africa and Europe, and has been developed to include chloride, alkalinity and electric conductivity. The weight assigned represents the value of the specific parameter, which has a significant influence on the WQI. In fact, an alternative WQI approach same as to Horton’s index was produced by some investigators Brown et al., (1970) that were based on parameter’s weight. Newly, numerous changes have been considered for WQI tools by many experts and researcher (Dwivedi et al. 1997; Bhargava et al. 1998).
GIS “Geographic Information System” is continuously developing technology and needs more efficient data processing and expertise solutions for the unified models of environmental simulation. Developments in methods for accessing and generating data such as internet, Global Positioning Systems along with Remote Sensing (Sweeney 1997). Proper use of ground water, which is precious but shrinking natural resource, requires a systematic plan of tapping using modern techniques. The advanced computer based and efficient methods for water management have evolved, of which RS, GIS and GPS are of great significance (Chowdhury et al. 2008). GIS may be a significant tool in order to develop resolutions for issues of water resources in order to manage resources of water on a regional scale, understanding the natural environment, preventing flooding, determining water convenience, and evaluating the quality of water (C Collet 1996).
The enhanced spatial and spectral resolution sensors as well as geospatial modeling approaches, water quality constraints like suspended particles, turbidity, algae bloom, chlorophyll, as well as mineral content in water bodies such as groundwater are tested at low cost and with greater precision (Ramadas and Samantaray 2018). The space–time groundwater content study based on GIS was conducted by (Singh et al. 2011b) utilizing the ArcGIS 9.1 Spatial Observer module. Awareness on physicochemical physical distribution and groundwater depth is an important need for water production and management (Gupta and Sarma 2016).
The key motive of this current investigation is for estimating the groundwater quality for drinking purpose using Geographical Information System (GIS) as well as Remote Sensing (RS) approaches coupled with water quality indexing related to significant physicochemical constraints of the groundwater from Bhokardan area of Jalna district, Maharashtra, India.
Study area
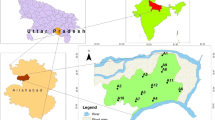
Bhokardan is a city and municipal council situated on the side of the River Kelana in Jalna District of Maharashtra State, India. Geographically, research area is situated between 21.27°N and 75.77°E. 1276 sq.km is a total geographical area along with average 587 m elevation (1925 feet). The sampling sites and research area are presented in the rainy season temperature varies between 21 and 30 °C. The temperature drops considerably in winter and is about 10 and 25 °C. Bhokardan taluka’s total annual precipitation is 641.6 mm. Farming in the region primarily relies on the south-west monsoon precipitation. The sub-tropical and tropical temperate monsoon climate are commonly experienced by the area. The amount of rainfall across the region was observed to be more or less consistent. This has been researched because much of the study area is landed on agriculture. In the northern portion, the forest covers much less region. Wherever developments exist, the build-up region is mirrored.
In the present study area, ground water plays an important role for irrigation and drinking. In the state of Maharashtra more than 70% of the rural areas are dependent on groundwater. In the same manner, more than 55% of the gross irrigated land of the state is subterranean. Several issues has been reported regarding quality of ground water by (WRD 2007a, b; CGWB 2016). Therefore, groundwater has an essential part in the economy of the state and thus required to be observed systematically both in quality and quantity to achieve sustainable management and development. Hence, this is essential to conduct a comprehensive study of ground water quality for management and proper identification of drinking water sources in this research area.
Materials and methods
Sample collection and analysis
The investigation of quality of ground water of Bhokardan region of Jalna district was undertaken during 2014–15. Total 35 integrated groundwater samples have been collected from open wells within radius of five km form main sampling points in post-monsoon as well as pre-monsoon seasons. These open wells have been being generally utilized for drinking and irrigation purpose. Sampling has been performed utilizing acid washed and pre-rinsed polyethylene vessels. The location of sampling points was recorded by GPS at each location. The water samples collected have been examined for EC (electrical conductivity), pH, Alkalinity, Turbidity, Conductivity, Total hardness, TDS (Total Dissolved Solids), Nitrate, Fluoride, Chloride, Phosphate, Sulphate along with MPN in laboratory utilizing these standard techniques represented by “American Public Health Association” (APHA 1998; CPCB 2011; Maiti 2011). In order to estimate DO (dissolved oxygen), Samples have been congregated in glass (BOD) bottles with stopper separately. The temperature of water has been noted on the site. The outcomes have been evaluated based on quality standards of drinking water stipulated by “Bureau of Indian Standards” (BIS 2012).
Calculation of Water Quality Index
WQI “Water Quality Index” has been estimated in three steps utilizing weighed arithmetic index approaches (Ramakrishnaiah et al. 2009). Firstly, selected 12 water quality parameters are very important in assessment of drinking water quality as well as has been assigned a wi (weight) shown in Table 1. The maximum weight of 5 was allocated to parameter MPN and Nitrate because of their significant role in WQI. Total hardness as well as potassium, which are performed the minimum weight of 2 since it, could not be harmful to human health. In next step, Wi (relative weight) is calculated by using the next formula.
Here, relative weight is represented by Wi, weight of each selected water quality parameter is described by wi, number of water quality parameters is represented by n. Table 1 represents the calculated Wi (relative weight) values of every water quality parameter.
In third stage, qi (quality rating scale) for individual water quality parameter is computed by dividing it’s observed value in respective water sample by its corresponding standard as per the drinking water guidelines prescribed by the BIS (2012) and the outcome is multiplied by 100.
Here, quality rating is defined by qi, concentration of individual chemical (Ci) characteristics in every water sample in mg/l, as well as Si is the Indian standard of drinking water for individual chemical characteristics in mg/l as per (BIS 2012) guidelines.
Firstly, SI is determined for computing WQI for every chemical parameter by multiplying quality rating with relative weight, finally the summation of sub-index is utilized to calculate WQI according to the given formula
Here, sub index (SIi) is the ith water quality parameter; the rating qi depends on concentration of ith parameter along with number of water quality parameters (n) undertaken for calculating GWQI. GWQI values are organized in 5 categories, “Excellent water” to “Water, unsuitable for drinking” as per Table 2.
Data used
A different data product required for the study includes Survey of India toposheets 46O/8, on 1:2,00,000 scales have been referred during interpretation as well as for base map preparation. The research area has been defined using Toposheets of 1:2,00,000 by identifying the study area boundaries by Scanning, projecting, geo-referencing and digitizing toposheets of the area manually using ArcGIS. Base map has been organized by visual interpretation utilizing toposheets as well as satellite image data of LISS III sensor with a spatial resolution of 23.5 m downloaded from open data archive (ISRO/NRSC 2019). Water samples have been taken from the area using integrated sampling techniques and the co-ordinates were noted down with GPS “Global Positioning System”.
Spatial modeling and surface interpolation through IDW
Spatial modeling (an extended module of ArcGIS 10.2) has been utilized for interpolating the spatial distribution of quality parameters of groundwater. IDW “Inverse Distance Weighted” interpolation approach has been utilized for creating various spatial distribution map of GWQI. IDW algorithm utilized for interpolating data spatially or evaluating of observations among measurements. Every observation has been projected in an IDW interpolation as well as weighted average of the neighboring sample points. The interpolation technique used by several investigator in their study was IDW (inverse distance weighted) techniques (Tabios and Salas 1985; Maciej Tomczak 1998; Mueller et al. 2004).
Results and discussions
Water is essential for life and all creatures residing on the earth need to provide a sufficient supply. The physical effects will come from purifying exposure to clean drinking water. Each attempt will be taken to ensure as healthy as practicable drinking water. Health experts are vulnerable to becoming mindful of a variety of clients in a large amount of drinking water sources in the majority of nations, whether developed countries or progressive countries. Nevertheless, as contaminants causing elevated risk to safety become common, there may be serious complications, even emergencies, but their prevalence remains uncertain, because their long-term health consequences arise from persistent and not acute exposure. These occurrences in groundwater arsenic in Bangladesh and the State in Bengal in India have been reported (WHO 2017a).
Assessing the quality of groundwater is significant due to its important aspect determining suitability for industrial use, agricultural, domestic, and drinking (Subramani et al. 2005). The main sources of groundwater pollution such as, agricultural area, landfills, hazardous waste disposal sites, sewerage systems, with high amounts of unabsorbed fertilizers, pesticides and other contaminants (Pal 2017). Water quality is affected by natural as well as human induced actions like irrigation, geology and local climate. Due to the enhancement in industrialization and urbanization, quality of groundwater decreases rapidly (Srinivas et al. 2000). Human health is affected strongly by quality of water (Amiri et al. 2014).
Physicochemical characteristics of groundwater samples in Bhokardan area has been examined statistically as well as outcomes like standard deviation, mean, minima, and maxima parameters are depicted in Table 3. The chemical and physical parameters of the analytical outcomes of groundwater have been compared to BIS guideline Standards for public health as well as drinking standards (BIS 2012).
Turbidity
In water, Turbidity is produced by suspended particulate matter like plankton, inorganic and organic matter, silt, clay, as well as another microscopic organisms or colloidal matter, which obstructs light transmission in water (Kavanaugh, M. C. 1979; APHA 1998). Below 4 NTU, Turbidity may be estimated only utilizing measuring instruments, however above 4 NTU, a whitish, black or brown, muddy, suspension may be observable. Turbidity is also a significant aesthetic water quality characteristics, with turbidity of 4 NTU and above affecting the appearance and suitability of drinking water to end users (WHO 2011a, 2017a; b).
Turbidity values of the groundwater samples were observed between 0.1 and 4.4 NTU and 5 24 NTU and throughout both post-monsoon and pre-monsoon season, respectively. According to (BIS 2012) permissible limit of the turbidity is 1 NTU. All turbidity values obtained during pre-monsoon period were beyond permissible limit whereas 26% of locations showed results beyond permissible limit during post-monsoon period. Turbidity above 5 NTU is undesirable for aesthetic reasons. Groundwater turbidity can be because of inorganic materials like manganese and iron. Excess of such inorganic substances are considered undesirable because it has adverse effect on water distribution and sanitation systems (Akhtar et al. 2014). Turbidity level should be maintained between 1 and 5 NTU to keep desirable drinking water quality (LeChevallier et al. 1981).
pH
In an aqueous solution, pH is controlled by interconnected chemical reactions that release or consume hydrogen ions (Hem 1985). There is no suggested pH safety guidance. Although pH value does not generally effect on users directly, it is one of the most significant constraint in terms of work water quality (WHO 2011b). The pH determines freshwater acidity and alkalinity. The volume and chemical composition of both organic and inorganic compounds contained in groundwater are primarily tracked (Sadat-Noori et al. 2014). In the current investigation, pH values of 97 percent of the samples have been within the permissible limits of BIS standards (BIS 2012), whereas 3 percent of sample showed beyond the permissible limit. It varies between 5.9 and 8.0 and 6.1 to 7.9 with an average value of 7.5 and 7.4 during both seasons, respectively. Average values of pH representative slightly alkaline nature of groundwater except for two sampling sites near Shipora village show acidic in nature for both seasons. This may be because of pollution of ground water through seepage of wastewater from the nearby sugar industry. The typically pH of the sugar industry effluent was in acidic range (< 5.5) as studied by (Poddar and Sahu 2017; Sahu 2017; Tiwari and Sahu 2017).
Total dissolved solids
“Total dissolved solids” (TDS) include inorganic salts, mainly, chlorides, bicarbonates, magnesium, calcium, sodium, potassium, sulphate as well as dissolved amount of organic matter in water. The various natural sources includes soil, rock and water interaction, and anthropogenic sources includes discharge of untreated sewage, industrial wastewater and urban runoff are main contributors of TDS in drinking water (WHO 2017a). Total dissolved solids are common constituent of several effluents, but are typically not well defined in terms of either chemical components or toxicity (Chapman et al. 2000). Water with higher TDS used for drinking purpose, consumers often reported to have a poor taste (Young et al. 1996).
TDS Concentrations from natural sources were observed to differ from < 30 to 6000 mg/L (WHO 1996). In the present study, for water samples TDS values vary between 442 and 985 mg/L having 668 mg/ average value in pre-monsoon season, whereas in the post-monsoon water samples range vary from 356 to 850 mg/L with an average of 581 mg/l. Maximum value of 985 mg/L has been seen at near Shipora village while minimum value of 442 mg/L has been detected at village Hasnabad in pre-monsoon season. Maximum value of 850 mg/L was observed at near Shipora village while minimum value of 356 mg/L has been detected at village Kumbhari in post-monsoon season. As per drinking water standards (BIS 2012) about 60 and 88% of samples have been discovered above permissible limit in both seasons, respectively. As per classification of water quality based on TDS concentration (WHO 1996), only 34% groundwater samples were fell into good (300 to 600 mg/L), whereas 3 and 63% fell into poor (900–1200 mg/L) and fair (600–900 mg/L) category, respectively, not even a single sample displayed excellent water quality (Less than 300 mg/L) for pre-monsoon season. During post-monsoon season 63% groundwater samples have been fell into good category, whereas 37% fell into fair category, none of the sample showed excellent as well as poor water quality as far as concern of TDS.
Electrical conductivity
Conductance is a calculation of the electric flow capability of water that is specifically related to ion concentration in the water (EPA 2012). The greater the salinity amount, the less the oxygen absorbed. In sea water, the solubility of oxygen at the same temperature is around 20 percent greater than in freshwater (Miller et al. 1988). An abrupt rise or decline in conductivity in the water body may indicate pollution. Sewage discharge or Agricultural runoff will rise conductivity because of addition of anions like chloride, phosphate and nitrate (EPA 2012). Diurnal electrical conductivity differences have also been reported during low-flow cycles (Hayashi et al. 2012) and it has also been suggested that EC shifts have been triggered by the mixture of chemical and biological processes.
In the present investigation values observed for conductivity during pre-monsoon period ranged from 726.3–1714 µS/cm at 36 °C, the minimum conductivity values were observed at village Hasnabad and Kumbhari with 726.3 and 740.3 µS/cm, respectively. Conductivity of the groundwater for the entire study area stands at an average of 1145 μS/cm. This data reveals that, there is high solute dissolution in groundwater, slow ion-exchange between soil as well as water, or basically soluble geologic rock and mineral forms in the area. There have been significant period changes in EC, which indicate higher values throughout pre-monsoon than after post-monsoon seasons that can be due to the high evaporation rate during dry season. During post-monsoon months, lower conductivity levels are attributed to precipitation dilution. Water leakage from the surface raises water concentration in the water body during pre-monsoon season and increases electric conductivity (Mandal et al. 2019).
Total hardness
Hardness is defined as the water capacity in order to react with water, but for producing a lather, hard water required significantly extra soap (WHO 2009). As per Indian standards, in water the limit for total hardness for human consumption (BIS 2012) is 500 mg/L. In this current research work, the observed total hardness range was 60 to 134 mg/L and 67–192 mg/L in both seasons, respectively. Every research locations for post as well as pre-monsoon period showed results for total hardness well below the standard limit as per Bureau of Indian Standard. The total hardness amount reported in the pre-monsoon season was found to be above that in the post-monsoon season. Water is classified as per contain of hardness. Calcium carbonate present in water at below 60 mg/L concentrations is usually called as soft water; concentrations more than 180 mg/L is known as very hard; 120 to 180 mg/L, hard; 60 to 120 mg/L, moderately hard (McGowan 2000). As per these classifications the ground water under studied for pre-monsoon period, 80% of locations placed in the category of moderately hard while 14% and 3% showed hard and very hard category, respectively. Almost 86% of groundwater samples out of 35 collected fall into moderately hard category whereas 9% and 5% fall into hard and soft category, respectively, for pre-monsoon period. Magnesium and Calcium dissolution from natural sources are removed to such a large degree that basalt cover the most of regions of Maharashtra and the capacity for sulphate and chloride from aquifers in groundwater is very small. This can be discharged from anthropogenic sources into groundwater (WRD 2007b).
Chloride
The salts such as NaCl (sodium), KCl (potassium) and CaCl2 (calcium), are chloride containing and commonly spread in nature. The weathering cycle absorbs chlorides from several rocks into the water and soil (WHO 1996). Based on the following, cation is the sensation of taste threshold of chloride anion in water. Sense of perception thresholds for sodium and calcium chloride in water are in the array 200–300 mg/L (Zoeteman BCJ 1980). The anthropogenic sources of chlorides in groundwater is inorganic fertilizer made with potash, sewage contamination and industrial effluents landfill leachates, animal fodders, irrigation drainage. Sodium chloride is broadly used in the manufacturing in industries to producte useful chemicals like; sodium hypochlorite, sodium chlorite, chlorine, and caustic soda. Magnesium chloride, calcium chloride and sodium chloride, are widely utilized in ice and snow control. Potassium chloride is utilized for the manufacturing of fertilizers (Department of National Health and Welfare (Canada) 1978; Mullaney et al. 2009; Kelly et al. 2012; Perera et al. 2013; Granato et al. 2015). Chlorides are essential for observing the contamination of ground water by wastewater. In this present study, the maximum value of Chlorides, i.e., 298.1 mg/L has been observed at village Anwa, whereas minimum value of 54.2 mg/L has been observed at village Gokul in post-monsoon period. The maximum value of 521 mg/L has been seen at Bhokardan, whereas minimum value of 98 mg/L has been observed at village Kumbhari in pre-monsoon season.
In post-monsoon, the average value of chloride is higher than to pre-monsoon because of increasing level of water which dissolves more salt from soil in post-monsoon periods (Ramesam 1982; Ballukraya PN 1999). Chloride is required to be below 250 mg/L in drinking water (BIS 2012). As per drinking water standards about 37% and 8.5% of samples have been found above permissible limit in both seasons, respectively. The presence of chloride in slightly higher amounts at some sites can be because of both natural as well as anthropogenic procedures such as passageway of water through natural salt formations in the beneath the ground and discharge of untreated sewage (Renn 1970). The physical processes like intercourse with other aquifer of diverse chloride concentration as well as change in evaporation rate throughout recharge moreover temporally or spatially can cause the changes in the chloride content of groundwater (Kumar et al. 2006). At crystalline rock sites chloride shows the most significant increase of all ions with increasing deepness in groundwaters (Frape et al. 2003).
Fluoride
Fluorides are available in many waters, but mostly observed at higher concentrations in the groundwaters. The fluoride concentration in natural water varies from 0.01 to 7.20 mg/l. Many areas of the world are abundant in fluoride content, well water can be contained fluoride at a concentration of about 10 mg/l. In several areas of the world, particularly areas of Americas, Central Africa, China, and India high fluoride levels can be found, although in other parts of the world there are lower concentrations. For groundwater, the fluoride amounts are derived from various rock from which the water runs but typically may not reach 10 mg/l; highest natural level reported is 2800 mg/l (Sloof et al. 1988; Gaciri and Davies 1993; Indermitte et al. 2009; Mohapatra et al. 2009; WHO 2011a; Katla and Saxena 2016; Kumar et al. 2019; Panda et al. 2019). Constant water–rock percolations of fluorite-bearing rocks in arid, low precipitation and high temperature also contributed to an increased evaporation cycle which led to a large amount of fluoride in groundwater (Machender et al. 2014). Fluoride is primarily added to the drinking water by different geological process. Fluoride influences teeth greatly by preventing and minimizing tooth-worsening hazards at low concentrations (Arumugam 2010), while fluoride concentrations above 1.5 mg/l can induce fluorosis in drinking water (BIS 2012).
In the study area, fluoride was detected in almost all samples and its concentration ranges from 0.5 to 1.2 mg/l during pre as well as post-monsoon period that is within desirable limit for drinking usage. pH values of groundwater in the present study area are varies between 7 and 8.5, that gives favorable environments for fluoride enrichment. Dissolution of F bearing minerals (principally CaF2) in groundwater is favored by high pH values (Chen et al. 2017). The absorption of fluoride into soils and rocks may be liable, since normal leaching and weathering are taking place, for the large concentration of fluoride found in surface water (Goyit et al. 2018). In India, the principal cause of high fluoride rates is the weathering of primary rocks and the liquidation of fluoride-containing minerals, typically correlated with low calcium levels and high bicarbonate ions (Ayoob and Gupta 2006). Mamatha & Haware, (2013) also reported fluoride concentration ranged from 0.2 to 3.0 mg/L ground and surface water of Mysore, Karnataka, India. In groundwaters, the high level concentrations of fluoride was reported more than 20 developing and developed countries, including India, high fluoride concentrations were recorded where 19 areas of India have been faced with serious fluorosis issues (Meenakshi and Maheshwari 2006).
Nitrate
Nitrate is normally found in the terrestrial environment and is an essential nutrient of the plants. High nitrate level can be observed in both surface water and groundwater as a result of various agricultural and allied activities significantly which includes excess use of inorganic nitrogenous fertilizers and manures as well as disposal wastewater by specific industries (Li et al. 2020). Degradation of nitrogenous waste products in animal and human excreta, such as septic tanks significantly add nitrates to the water. Surface water nitrate levels may rapidly increase due to surface rinse, phytoplankton absorption and nitrate denitrification by bacteria, but groundwater nitrate levels typically exhibit slow changes. Groundwaters may also have nitrate contamination as a result of leaching from natural foliage (WHO 2011c). The concentration of nitrogen in groundwater is also derived from the biosphere (Saleh A, Al-Ruwih F 1999). The chief source of nitrate in groundwater is leaching of nitrate with the permeating water due to the numerous agriculture and anthropogenic activities. Groundwater can also be contaminated by sewage and other wastes rich in nitrates (Amiri et al. 2014; CGWB 2014). The shallow groundwater samples had nitrate resulting mostly from soil nitrification. Sewage and manure also contribute nitrate in ground water (Rock and Mayer 2002). Almost all the cases of excessive nitrate build-up in shallow ground waters were due to the downward leaching of nitrogen by surface water as reviewed in report of CGWB (2014).
In the study area, nitrate concentration varies from 28.5 to 145.7 mg/l and 22.9 to 234.9 mg/l with an average value of 64.7 and 108.2 mg/l for pre-monsoon and post-monsoon period, respectively. As per Bureau of Indian Standards, drinking water standard for nitrate is 45 mg /l. The investigation revealed that, 85 and 92% of ground water sources of the study area showed nitrate level beyond the maximum allowable limit for drinking purpose in pre-monsoon and post-monsoon period, respectively. (Wagh et al. 2020) observed that 52.5 and 65% groundwater samples groundwater from Kadava River Basin Nashik, Maharashtra, India during pre and post-monsoon period was unfit for drinking due to of high nitrate concentration exceeding the permissible limit of nitrate (> 45 mg/l) recommended by the (BIS 2012). The reviewed studies by (Gupta et al. 2011) revealed that nitrate pollution is becoming more predominant in groundwater of Maharashtra. Water quality data during the period 2007–2009 depicted those 544 locations out of 1407 locations exceeded the permissible limit of 45 mg/l, for drinking purpose prescribed by BIS. Higher level of nitrate might have been either due to overuse of fertilizers or due to leaching (Anku et al. 2009; Singh et al. 2010). Increasing concentration of nitrate were associated with greater well depth and higher percentage of agricultural land use (Kent and Landon 2013). According to fact sheet of the European Union (EU) on agri-environmental indicator water quality nitrate pollution, there was declining trends in groundwater nitrate typically be due to improvements in the management of agricultural field (EUROSTAT 2012). Individual wells in agricultural zones throughout the world particularly contribute to nitrate related toxicity problems and nitrate levels in the well water often exceed 50 mg/l (WHO 2011c). The high concentration of nitrate in drinking water has adverse effects on human health and causes and gastric carcinomas and blue baby syndrome in children which is commonly known as Methemoglobinemia (Comly 1945; Gilly G, Corrao G 1984). The concentration of nitrate in the present study was observed higher than those reported by (Jinwal and Dixit 2008; Boateng et al. 2016).
Sulphate
Sulphate occur naturally in several minerals and are used commercially, mainly in the various chemical industries. They are discharged into water by industrial wastes and through atmospheric deposition; though, the highest levels generally occur in groundwater mostly are from natural sources. In addition, groundwater that has been polluted with fertilizer and industrial effluent may also comprise ammonium sulphate (Ingham 2013). Taste enhancement varies with the properties of the associated cation; taste thresholds have been found to range from 250 mg/l for sodium sulphate to 1000 mg/l for calcium sulphate. It is commonly considered that taste impairment is minimal at levels below 250 mg/l. No health-based guideline value has been derived for sulphate (WHO 2011a; Krishna kumar et al. 2015).
In ground water of the study area, concentrations of sulphate varied from 11.5 to 139.3 mg/l and 11.28 to 140.5 mg/l with an average level of 79.32 and 77.12 mg/l during pre-monsoon and post-monsoon period, respectively. The observed levels of sulphate in all groundwater samples from the study area was well below permissible limit of 200 mg/l and 250 mg/l prescribed by (BIS 2012) and (WHO 2017a), respectively. Sulfur is generally present in reduced forms in igneous, sedimentary and metamorphic rocks as metallic sulfides. This sulfur converted to sulphate when weathered in contact with aerated water. Water in igneous and metamorphic rocks usually comprises less than 100 mg/L sulphate, but sedimentary rocks can contain much higher levels of sulphate (Fytianos and Christophoridis 2004).
Groundwater samples are undersaturated with respect to gypsum and gypsum dissolution, thus leading to an increased level of calcium and sulphate along groundwater flow direction (Bucher K 2000; Ingham 2013; Nassery and Kayhomayoon 2013). Other than this, overuse of sulphate fertilizers and surface runoff from agricultural field can be considered as a secondary source of increasing sulphate concentration in groundwater (Amiri et al. 2014). Irrigation water sources had a significant effects on the increase in the level of sulphate at the shallow groundwater, particularly when groundwater with elevated concentrations was used for irrigation (Liu et al. 2013).
Potassium
Potassium is an important element required in humans. Potassium generally available in the environment, including all natural water resources. It can also observe in drinking water due to the application of potassium permanganate as an oxidant in water treatment process. It is not considered essential to establish a health related standard value for potassium in drinking water while potassium may cause some health effects in susceptible individuals, potassium intake from drinking water is well below the level at which antagonistic health effects may occur (WHO 2017a). In the present investigation, Potassium concentration ranges from 3.7 to 20.3 mg/l with an average value of 8.1 mg/l during pre-monsoon period and 0.5 to 10.9 mg/l with an average value of 3.13 mg/l during post-monsoon period. As per (WHO 2004), the maximum allowable limit for potassium is 10 mg/l. Potassium concentrations were higher in the pre-monsoon period compared to the post-monsoon season. The concentration of potassium at a most of locations is unusually within permissible limit. Comparable results were observed by (Singh et al. 2011a, b; Shanmugasundharam et al. 2017). The higher concentration of potassium in ground water may be due to salt patches present geogenically and fertilizer leaching or percolating through the sub-surface (Selvam et al. 2014). The potassium level observed in the groundwater samples may be due to the weathering of potassium bearing minerals or some cation-exchange processes and agricultural activities (Guo and Wang 2004; Singh et al. 2006).
Escherichia coli
E. coli is present in large numbers in the normal intestinal flora of humans and animals, where it normally causes no harm. Though, in other parts of the body, E. coli can be responsible for serious illness, such as urinary tract infections, bacteraemia and meningitis. Total coliforms can also live and grow in water distribution systems, mostly in the presence of biofilms (WHO 2011a). As per (WHO 1997; BIS 2012) E. coli should not be present in at all 100 ml sample. In the present study, E. coli is determined by multiple tube (Most Probable Number) method. It was significantly detected at 37% and 71% of locations during pre-monsoon and post-monsoon period, respectively. The highest values of E. coli may be attributed to surface runoff from urban areas and percolation process, signifying that the bacteriological water in these areas was not safe and required treatment before drinking. The highest faecal coliforms 16 and 26 was observed at Bhokardan in pre and post-monsoon season, respectively. Bhokardan city is the only part of the study area which falls under urban site. Studies were conducted in groundwater by (Cook and Bolster 2007) concluded that Escherichia coli survival was the greatest when the total dissolved nitrogen (12 mg/l) was high. Similarly, high nitrate level was detected in the present study area which may be reason for observed values of E. coli.
Water Quality Index (WQI)
Water quality index is one of the most successful tools to represent information on the quality of streams, lakes or any water body. WQI is a scientific equation used to transform large number of water quality data into a single number which is easily understandable to lay man (Štambuk-Giljanović 1999; Tyagi and Sharma 2014). It serves the understanding of water quality issues by integrating complex data and generating a score that describes water quality status (Reza and Singh 2010). WQI value is computed for the suitability of surface and groundwater for domestic uses human (Sahu and Sikdar 2008; Ravikumar et al. 2011). To get a complete picture of the quality of the groundwater, water quality index is one of the most operative tools (Tiwari, TN and Mishra 1985). WQI tool has effectively employed by various investigators worldwide (Soltan 1999; Khan et al. 2003; Singh et al. 2006, 2011b; Sahu and Sikdar 2008; Ramakrishnaiah et al. 2009; Srivastava et al. 2012; Ketata et al. 2012; Pei-Yue et al. 2012; Abou Zakhem and Hafez 2015; Boateng et al. 2016; Shah and Joshi 2017; Şener et al. 2017; Dandge et al. 2018; Kumari and Sharma 2018).
For the present investigation calculated WQI values are classified into five types namely, Excellent water (WQI < 50), Good water (50 < WQI < 100), poor water (100 < WQI < 200), very poor Water (200 < WQI < 300) and water unsuitable for drinking purpose (WQI > 300). The water quality index data of the present study revealed that, 17% groundwater sources were of good quality, 40% had poor water quality, 29% exhibited very poor water quality and 14% showed water unsuitable for drinking purpose during pre-monsoon season while 62% groundwater sources were of good quality, 20% had poor water quality, 9% exhibited very poor water quality and 9% showed water unsuitable for drinking purpose during post-monsoon season. The high WQI values in groundwater samples were principally due to the occurrence of higher values of turbidity and E. Coli. The higher concentration of water quality parameters like total dissolved solids, electrical conductivity, total alkalinity, potassium, total hardness, fluoride and chloride also accountable for high WQI values in the study area. None of the location falls under excellent water quality during pre and post- monsoon period. The study reveals that the quality of groundwater was better during the post-monsoon season, as compared to that of pre-monsoon season. Similar results were observed by (Khan et al. 2015). The study reveals that the quality of groundwater was far better during the post-monsoon season, as compared to that of pre-monsoon season (Figs. 1, 2 and 3).
Figures 4 and 5 shows the spatial distribution of GWQI in the study area, based on inverse distance weighting (IDW) interpolation. The spatial map depicted that all part of the study area falls under the category of “Poor water” and very poor water except villages Padmavati, Wadhona and Jaidevwadi at Northern part of the area which falls under the category of “Good water” during pre-monsoon, whereas most of sites from Northern and Southern part of the region shifted their category from poor to good water and very poor and poor water during post-monsoon season. As per WQI data, worse quality of ground water was observed at Bhokardan and near Shipora village in pre and post-monsoon season. seasons. Analogues trends were observed by several investigator (Jinwal and Dixit 2008; Rajankar et al. 2011; Dutta and Singh 2015; Khan et al. 2015; Sharma et al. 2017; Verma et al. 2018, 2020). None of the site falls under the excellent category during both seasons in present study area.
Conclusion
Groundwater quality and its suitability for drinking purposes in Bhokardan area has been evaluated. The water quality index data of the present study revealed that, 17% groundwater sources were of good quality, 40% had poor water quality, 29% exhibited very poor water quality and 14% showed water unsuitable for drinking purpose during pre-monsoon season while 62% groundwater sources were of good quality, 20% had poor water quality, 9% exhibited very poor water quality and 9% showed water unsuitable for drinking purpose during post-monsoon season. The high WQI values in groundwater samples were principally due to the occurrence of higher values of turbidity and E. Coli. The higher concentration of water quality parameters like total dissolved solids, electrical conductivity, total alkalinity, potassium, total hardness, fluoride and chloride were also accountable for high WQI values in the study area. E. coli was significantly detected at 37% and 71% of locations during pre-monsoon and post-monsoon period respectively, therefore it is recommended that, not to consume water without proper treatment. Ground water quality is badly affected at many places due to high concentration of some parameters, especially nitrate and E. coli. Suitable sanitary defense to the wells used as a source of drinking water may be provided to control the nutrients’ contamination. The spatial distribution map showed that all parts of the study area falls under the category of “Poor water” and “Very poor water” except villages some sites at Northern part of the area which falls under the category of “Good water” during pre-monsoon, whereas most of the sites from Northern and Southern part of the region shifted category from poor to good water and very poor to poor water during post-monsoon season. Worse situation was observed at centrally located sites near Shipora village and Bhokardan. Significantly, minimum WQI for the post-monsoon season was observed as compared to pre-monsoon season, demonstrating worsening overall quality of the groundwater in corresponding season. The study revealed that, groundwater from only 17% locations was good for domestic use in pre-monsoon season, thus signifying the need of suitable treatment before use it for drinking purpose. In conclusion, from the outcomes of the present study it may be said that exhaustive groundwater analysis should be conducted frequently to monitor the rate and kind of pollution. It is need of human to expand awareness among the people to maintain the groundwater at their highest quality, and purity levels. The higher values of WQI observed in the numerous groundwater samples of the Bhokardan area of Jalna district, indicated that water is not suitable for direct drinking, and it is essential to apply sustainable treatment of raw water by various appropriate physical and chemical processes before it’s utilization for drinking purpose.
References
APHA (1998) Standard Methods for the Examination of Water and Wastewater, 20th Edition. American Public Health Association
Abou Zakhem B, Hafez R (2015) Heavy metal pollution index for groundwater quality assessment in Damascus Oasis, Syria. Environ Earth Sci 73:6591–6600. https://doi.org/10.1007/s12665-014-3882-5
Akhtar MM, Tang Z, Mohamadi B (2014) Contamination potential assessment of potable groundwater in Lahore, Pakistan. Polish J Environ Stud 23:1905–1916
Amiri V, Rezaei M, Sohrabi N (2014) Groundwater quality assessment using entropy weighted water quality index (EWQI) in Lenjanat. Iran Environ Earth Sci 72:3479–3490. https://doi.org/10.1007/s12665-014-3255-0
Anku YS, Banoeng-Yakubo B, Asiedu DK, Yidana SM (2009) Water quality analysis of groundwater in crystalline basement rocks, Northern Ghana. Environ Geol 58:989–997. https://doi.org/10.1007/s00254-008-1578-4
Arumugam K (2010) Assessment of groundwater quality in Tirupur Region. Anna University, Chennai
Awodumi OE, Akeasa OS (2017) GIS applications for assessing spatial distribution of boreholes and hand dug wells in Boroboro community, Atiba Local Government. Oyo State J Remote Sens GIS. https://doi.org/10.4172/2469-4134.1000208
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487. https://doi.org/10.1080/10643380600678112
BIS (2012) Indian Standard, DRINKING WATER-SPECIFICATION ( Second Revision ). Bureau of Indian Standards, New Delhi, New Delhi
Balakrishnan P (2012) Groundwater quality mapping using geographic information system (GIS): a case study of Gulbarga City, Karnataka, India. African J Environ Sci Technol 5:1069–1084. https://doi.org/10.5897/ajest11.134
Ballukraya PNRR (1999) Characterization of groundwater in the unconfined aquifer of Chennai City, India. J - Geol Soc India 54:1–11
Bhardwaj RM (2005) Water Quality monitoring in India- achievements and constraints. International Work Session on Water Statistics. Austria, Vienna, pp 1–12
Bhargava D, Saxena BS, Adewakar RW (1998) A study of geo-pollutants in the Godavary river basin in India. Asian Environ 12:36–59
Boateng TK, Opoku F, Acquaah SO, Akoto O (2016) Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality, Ghana. Environ Earth Sci 75:1–14. https://doi.org/10.1007/s12665-015-5105-0
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) Water quality index-do we dare? Water Sew Work 117(339):343
Bucher KSI (2000) Hydrochemistry of water in the crystalline basement. Kluwer Academic Publishers, Dordrecht, The Netherlands
CGWB (2014) Concept Note On geogenic contamination of ground water in India Faridabad
CGWB (2016) Report on aquifer maps and ground water management plan bhokardan Taluka, Jalna District, Maharashtra Part-I
CPCB (2011) Guide manual: water and wastewater analysis. Central Pollution Control Board
Chapman PM, Bailey H, Canaria E (2000) Toxicity of total dissolved solids associated with two mine effluents to chironomid larvae and early life stages of rainbow trout. Environ Toxicol Chem 19:210–214. https://doi.org/10.1002/etc.5620190125
Chen J, Wu H, Qian H, Gao Y (2017) Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of Northwest China. Expo Heal. https://doi.org/10.1007/s12403-016-0231-9
Chowdhury A, Jha MK, Chowdary VM, Mal BC (2008) Integrated remote sensing and GIS-based approach for assessing groundwater potential in West Medinipur district, West Bengal, India. Int J Remote Sens 30:231–250. https://doi.org/10.1080/01431160802270131
Collet C (1996) Geographic information system needs and software. Kluwer Academic Publishers, USA, Boston
Comly H (1945) Cyanosis in infants caused by nitrates in well water. J Am Med Assoc 129:12–144
Cook KL, Bolster CH (2007) Survival of Campylobacter jejuni and Escherichia coli in groundwater during prolonged starvation at low temperatures. J Appl Microbiol 103:573–583. https://doi.org/10.1111/j.1365-2672.2006.03285.x
Dandge K, More G, Ghope N (2018) Geospatial technology for mapping spatial variations of Ground Water Quality Index in Erandol area of Jalgaon District, Maharashtra State, India. Int J Recent Sci Res 9:25274–25279
Department of National Health and Welfare (Canada) (1978) Guidelines for Canadian drinking water quality. Ottawa
Dutta S, Singh S (2015) Seasonal variation in different physico-chemical characteristics in ground water quality of Pali industrial area, Rajasthan, India. Int J Res Pharm Sci 5:25–29
Dwivedi S, Tiwari IC, Bhargava DS (1997) Water quality of the river Ganga at Varanasi. Inst Eng Kolkota 78:1–4
EPA (2012) Conductivity In Water: Monitoring and Assessment
EUROSTAT (2012) Agri-environmental indicator - nitrate pollution of water - Statistics Explained Stat Off Eur Communities 1–11
Frape SK, Blyth A, Blomqvist R et al (2003) 5.17 - deep fluids in the continents: II. crystalline rocks. In: Holland HD, Turekian KK (eds) Treatise on Geochemistry. Pergamon, Oxford, pp 541–580
Fytianos K, Christophoridis C (2004) Nitrate, Arsenic and Chloride pollution of drinking water in Northern Greece Elaboration by Applying GIS. Environ Monit Assess 93:55–67. https://doi.org/10.1023/B:EMAS.0000016791.73493.aa
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412. https://doi.org/10.1016/0022-1694(93)90201-J
Gilly G, Corrao GFS (1984) Concentrations of nitrates in drinking water and incidence of gastric carcinomas First descriptive study of the Piemonate Region. Italy Sci Total Environ 34:35–37
Goyit MP, Solomon OA, Kutshik RJ (2018) Distribution of fluoride in surface and ground water: a case study of Langtang North, Plateau State, Nigeria. Int J Biol Chem Sci 12:1057. https://doi.org/10.4314/ijbcs.v12i2.33
Granato GE, DeSimone LA, Barbaro JR, Jeznach LC (2015) Methods for evaluating potential sources of chloride in surface waters and groundwaters of the conterminous United States:: U.S. Geological Survey Open-File Report 2015–1080, 89 p
Guo H, Wang Y (2004) Hydrogeochemical processes in shallow quaternary aquifers from the northern part of the Datong Basin China. Appl Geochem 19:19–27. https://doi.org/10.1016/S0883-2927(03)00128-8
Gupta I, Salunkhe A, Rohra N, Kumar R (2011) Groundwater quality in Maharashtra, India: focus on nitrate pollution. J Environ Sci Eng 53:453–462
Gupta P, Sarma K (2016) Spatial distribution of various parameters in groundwater of Delhi, India. Cogent Eng 3:1–10. https://doi.org/10.1080/23311916.2016.1138596
Hayashi M, Vogt T, Mächler L, Schirmer M (2012) Diurnal fluctuations of electrical conductivity in a pre-alpine river: effects of photosynthesis and groundwater exchange. J Hydrol 450–451:93–104. https://doi.org/10.1016/j.jhydrol.2012.05.020
Hem JD (1985) Study and interpretation of chemical characteristic of natural water US Geological Survey Water supply paper Vol 2254
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37:300–306
Hosseini Moghari SM, Ebrahimi K, Azarnivand A (2015) Groundwater quality assessment with respect to fuzzy water quality index (FWQI): an application of expert systems in environmental monitoring. Environ Earth Sci 74:7229–7238. https://doi.org/10.1007/s12665-015-4703-1
ISRO/NRSC (2019) Satellite Images LISS III. In: Open Data Arch. Bhuvan
Indermitte E, Saava A, Karro E (2009) Exposure to high fluoride drinking water and risk of dental fluorosis in estonia. Int J Environ Res Public Health 6:710–721. https://doi.org/10.3390/ijerph6020710
Ingham JP (2013) 5 - Concrete. In: Ingham JP (ed) Geomaterials under the microscope. Academic Press, Boston, pp 75–120
Jinwal A, Dixit S (2008) Pre- and Post-Monsoon variation in physico-chemical characteristics in groundwater quality of Bhopal " The City of Lakes " India. Asian J Exp Sci 22:311–316. https://doi.org/10.6088/ijes.2014040404515
Katla S, Saxena PR (2016) Analysis of Fluoride fluctuations in groundwater samples of pre-monsoon and post-monsoon seasons in and around 7:138–143
Kavanaugh MC and ART (1979) Measurement of turbidity In: Advances in laboratory techniques for quality control American Water Works Association, Denver, pp 41–53
Kelly WR, Panno SV, Hackley K (2012) The sources, distribution, and trends of chloride in the waters of Illinois. Prairie Research Institute University of Illinois at Urbana-Champaign Champaign, Illinois
Kent R, Landon MK (2013) Trends in concentrations of nitrate and total dissolved solids in public supply wells of the Bunker Hill, Lytle, Rialto, and Colton groundwater subbasins, San Bernardino County, California: Influence of legacy land use. Sci Total Environ 452–453:125–136. https://doi.org/10.1016/j.scitotenv.2013.02.042
Ketata M, Gueddari M, Bouhlila R (2012) Use of geographical information system and water quality index to assess groundwater quality in el khairat deep aquifer (enfidha, central east tunisia). Arab J Geosci 5:1379–1390. https://doi.org/10.1007/s12517-011-0292-9
Khan F, Husain T, Lumb A (2003) Water quality evaluation and trend analysis in selected watersheds of the atlantic region of Canada. Environ Monit Assess 88:221–242. https://doi.org/10.1023/A:1025573108513
Khan S, Khan S, Khan MN, Khan AA (2015) Pre and post monsoon variation in Physico-Chemical characteristics in groundwater quality of Shahjahanpur the town of Martyrs, India : a case study. Int Res J Environ Sci 4:107–114
Krishna Kumar S, Logeshkumaran A, Magesh NS et al (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5:335–343. https://doi.org/10.1007/s13201-014-0196-4
Kumar M, Ramanathan A, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50:1025–1039. https://doi.org/10.1007/s00254-006-0275-4
Kumar S, Singh R, Venkatesh AS et al (2019) Medical geological assessment of fluoride contaminated groundwater in parts of Indo-Gangetic alluvial plains. Sci Rep 9:1–16. https://doi.org/10.1038/s41598-019-52812-3
Kumari R, Sharma RC (2018) Assessment of water quality index and multivariate analysis of high altitude sacred Lake Prashar Himachal Pradesh India. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-018-2007-1
LeChevallier MW, Evans TM, Seidler RJ (1981) Effect of turbidity on chlorination efficiency and bacterial persistence in drinking water. Appl Environ Microbiol 42:159–167. https://doi.org/10.1128/aem.42.1.159-167.1981
Li X, Zhang Y, Wu T et al (2020) Major ions in drinking and surface waters from five cities in arid and semi-arid areas, NW China: spatial occurrence, water chemistry, and potential anthropogenic inputs. Environ Sci Pollut Res 27:5456–5468. https://doi.org/10.1007/s11356-019-07149-9
Liu X, Šimůnek J, Li L, He J (2013) Identification of sulfate sources in groundwater using isotope analysis and modeling of flood irrigation with waters of different quality in the Jinghuiqu district of China. Environ Earth Sci 69:1589–1600. https://doi.org/10.1007/s12665-012-1993-4
Machender G, Dhakate R, Narsimha Reddy M (2014) Hydrochemistry of groundwater (GW) and surface water (SW) for assessment of fluoride in Chinnaeru river basin, Nalgonda district, (AP) India. Environ Earth Sci 72:4017–4034. https://doi.org/10.1007/s12665-014-3291-9
Maiti S (2011) Handbook of methods in environmental studies, Volume 1: Water and Wastewater analysis. Oxford Book Company, Jaipur
Mamatha S, Haware D (2013) Document on Fluoride accumulation in ground and surface water of Mysore Karnataka India. Curr World Environ J 8:259–265. https://doi.org/10.12944/cwe.8.2.11
Mandal SK, Dutta SK, Pramanik S, Kole RK (2019) Assessment of river water quality for agricultural irrigation. Int J Environ Sci Technol 16:451–462. https://doi.org/10.1007/s13762-018-1657-3
McGowan W (2000) Water processing: residential, commercial, light-industrial, 3rd edn. Water Quality Association, Lisle, IL
Meenakshi and Maheshwari RC (2006) Fluoride in drinking water and its removal J Hazard Mater 137:456–463 https://doi.org/10.1016/j.jhazmat.2006.02.024
Miller RL, Bradford WL, Peters NE (1988) Specific conductance: theoretical considerations and application to analytical quality control. United States Geological Survey Water-Supply Paper 2311
Milovanovic M (2007) Water quality assessment and determination of pollution sources along the Axios/Vardar River, Southeastern Europe. Desalination 213:159–173. https://doi.org/10.1016/j.desal.2006.06.022
Mohapatra M, Anand S, Mishra BK et al (2009) Review of fluoride removal from drinking water. J Environ Manag 91:67–77. https://doi.org/10.1016/j.jenvman.2009.08.015
Mueller TG, Pusuluri NB, Mathias KK et al (2004) Map quality for ordinary Kriging and inverse distance weighted interpolation. Soil Sci Soc Am J 68:2042. https://doi.org/10.2136/sssaj2004.2042
Mullaney JR, Lorenz DL, Arntson AD (2009) Chloride in Groundwater and Surface Water in Areas Underlain by the Glacial Aquifer System , Northern United States: U. S. Geological Survey Scientific Investigations Report 2009–5086
Murthy KSR (2000) Ground water potential in a semi-arid region of Andhra Pradesh - a geographical information system approach. Int J Remote Sens 21:1867–1884. https://doi.org/10.1080/014311600209788
Nassery HR, Kayhomayoon Z (2013) Source of salinity in the groundwater of Lenjanat Plain, Isfahan. Iran Environ Earth Sci 68:413–427. https://doi.org/10.1007/s12665-012-1746-4
Pal P (2017) Chapter 1 - Introduction. In: Pal P (ed) Industrial water treatment process technology. Butterworth-Heinemann, pp 1–19
Panda B, Radha VD, Chidambaram S et al (2019) Chapter 22 - fluoride contamination in groundwater—A GIS and geostatistics reappraisal. In: Venkatramanan S, Prasanna MV, Chung SY (eds) GIS and geostatistical techniques for groundwater science. Elsevier, Amsterdam, pp 309–322
Pandian M, Jeyachandran N (2014) Groundwater Quality mapping using remote sensing and GIS – a case study at Thuraiyur and Uppiliapuram block. Tiruchirappalli 3:580–591
Pei-Yue L, Hui Q, Jian-Hua W (2012) Groundwater Quality assessment based on improved water quality index in Pengyang County, Ningxia, Northwest China. E-Journal Chem 7:S209–S216. https://doi.org/10.1155/2010/451304
Perera N, Gharabaghi B, Howard K (2013) Groundwater chloride response in the Highland Creek watershed due to road salt application: a re-assessment after 20years. J Hydrol 479:159–168. https://doi.org/10.1016/j.jhydrol.2012.11.057
Poddar PK, Sahu O (2017) Quality and management of wastewater in sugar industry. Appl Water Sci 7:461–468. https://doi.org/10.1007/s13201-015-0264-4
Rajankar PN, Tambekar DH, Wate SR (2011) Groundwater quality and water quality index at Bhandara District. Environ Monit Assess 179:619–625. https://doi.org/10.1007/s10661-010-1767-y
Ramadas M, Samantaray AK (2018) Applications of remote sensing and GIS in Water Quality Monitoring and remediation: a state-of-the-art review. In: Gupta AB, Gupta A, Pandey A (eds) Bhattacharya S. Springer Singapore, Singapore, pp 225–246
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009) Assessment of Water Quality Index for the groundwater in Tumkur Taluk, Karnataka State, India. E-Journal Chem 6:523–530
Ramesam V (1982) Geochemistry of groundwater from a typical hard rock terrain (Vedavati River Basin). J Geol Soc India 23:201–204
Ravikumar P, Venkatesharaju K, Prakash KL, Somashekar RK (2011) Geochemistry of groundwater and groundwater prospects evaluation, Anekal Taluk, Bangalore Urban District, Karnataka, India. Environ Monit Assess 179:93–112. https://doi.org/10.1007/s10661-010-1721-z
Renn CE (1970) Investigating water problems. Educational Products Division, LaMotte Chemical Products Company, Maryland
Reza R, Singh G (2010) Assessment of ground Water Quality status by using water quality index method in Orissa, India. World Appl Sci J 9:1392–1397
Rock L, Mayer B (2002) Isotopic assessment of sources and processes affecting Sulfate and Nitrate in surface water and groundwater of Luxembourg. Isotopes Environ Health Stud 38:191–206. https://doi.org/10.1080/10256010208033265
Sadat-Noori SM, Ebrahimi K, Liaghat AM (2014) Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer. Iran Environ Earth Sci 71:3827–3843. https://doi.org/10.1007/s12665-013-2770-8
Sahu O (2017) Treatment of sugar processing industry effluent up to remittance limits: suitability of hybrid electrode for electrochemical reactor. MethodsX. 4:172–185. https://doi.org/10.1016/j.mex.2017.05.001
Sahu P, Sikdar PK (2008) Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal, India. Environ Geol 55:823–835. https://doi.org/10.1007/s00254-007-1034-x
Saleh A, Al-Ruwih FSM (1999) Hydrogeochemical process operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Selvam S, Manimaran G, Sivasubramanian P et al (2014) GIS-based evaluation of Water Quality Index of groundwater resources around Tuticorin coastal city, south India. Environ Earth Sci 71:2847–2867. https://doi.org/10.1007/s12665-013-2662-y
Şener Ş, Şener E, Davraz A (2017) Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.01.102
Shah KA, Joshi GS (2017) Evaluation of water quality index for River Sabarmati, Gujarat, India. Appl Water Sci 7:1349–1358. https://doi.org/10.1007/s13201-015-0318-7
Shanmugasundharam A, Kalpana G, Mahapatra SR et al (2017) Assessment of groundwater quality in Krishnagiri and Vellore Districts in Tamil Nadu, India. Appl Water Sci 7:1869–1879. https://doi.org/10.1007/s13201-015-0361-4
Sharma DA, Rishi MS, Keesari T (2017) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in southwest Punjab, India using hydrochemical approach. Appl Water Sci 7:3137–3150. https://doi.org/10.1007/s13201-016-0456-6
Singh KP, Malik A, Mohan D et al (2006) Evaluation of groundwater quality in Northern Indo-Gangetic Alluvium region. Environ Monit Assess 112:211–230. https://doi.org/10.1007/s10661-006-0357-5
Singh C, Shashtri S, Mukherjee S (2011a) Integrating multivariate statistical analysis with GIS for geochemical assessment of groundwater quality in Shiwaliks of Punjab, India. Environ Earth Sci 62:1387–1405. https://doi.org/10.1007/s12665-010-0625-0
Singh CK, Shashtri S, Mukherjee S et al (2011b) Application of GWQI to assess effect of land use change on groundwater quality in lower Shiwaliks of Punjab: remote sensing and GIS based approach. Water Resour Manag 25:1881–1898. https://doi.org/10.1007/s11269-011-9779-0
Singh SK, Singh CK, Mukherjee S (2010) Impact of land-use and land-cover change on groundwater quality in the Lower Shiwalik hills: a remote sensing and GIS based approach. Cent Eur J Geosci 2:124–131. https://doi.org/10.2478/v10085-010-0003-x
Sloof W, Eerens HC, Janus JA, Ros JPM (1988) Basisdocument fluoriden. Bilthoven, Netherlands, National Institute of Public Health and Environmental Protection (Report No. 758474005)
Soltan ME (1999) Evaluation of ground water quality in Dakhla Oasis (Egyptian Western Desert). Environ Monit Assess 57:157–168. https://doi.org/10.1023/A:1005948930316
Srinivas CH, Piska RS, Venkateshwar C, Rao MSS, Reddy RR (2000) Studies on ground water quality of Hyderabad. Pollut Res 19:285–289
Srivastava PK, Gupta M, Mukherjee S (2012) Mapping spatial distribution of pollutants in groundwater of a tropical area of India using remote sensing and GIS. Appl Geomatics 4:21–32. https://doi.org/10.1007/s12518-011-0072-y
Štambuk-Giljanović N (1999) Water quality evaluation by index in Dalmatia. Water Res 33:3423–3440. https://doi.org/10.1016/S0043-1354(99)00063-9
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110. https://doi.org/10.1007/s00254-005-1243-0
Sweeney MW (1997) Geographic information systems. Wiley, Hoboken, pp 419–422
Tabios GQ, Salas JD (1985) A comparative analysis of techniques for spatial interpolation of precipitation. JAWRA J Am Water Resour Assoc 21:365–380. https://doi.org/10.1111/j.1752-1688.1985.tb00147.x
Tiwari TN, Mishra M (1985) A preliminary assignment of water quality index of major Indian rivers. Indian J Environ Prot 5:276–279
Tiwari A, Sahu O (2017) Treatment of food-agro (sugar) industry wastewater with copper metal and salt: chemical oxidation and electro-oxidation combined study in batch mode. Water Resour Ind 17:19–25. https://doi.org/10.1016/j.wri.2016.12.001
Tomczak M (1998) Spatial interpolation and its uncertainty using automated anisotropic inverse distance weighting (IDW) - Cross-Validation/Jackknife approach. J Geogr Inf Decis Anal 2:18–30
Tyagi S, Sharma B (2014) Water Quality assessment in terms of Water Quality Index Water Quality assessment in terms of Water Quality Index Water Quality Assessment in terms of Water Quality Index. Am J Water Resour 2013 1 (3): 34–38 1:34–38. https://doi.org/10.12691/ajwr-1-3-3
Verma DK, Bhunia GS, Shit PK, Tiwari AK (2018) Assessment of groundwater quality of the central Gangetic Plain area of India using geospatial and WQI techniques. J Geol Soc India 92:743–752. https://doi.org/10.1007/s12594-018-1097-1
Verma P, Singh PK, Sinha RR, Tiwari AK (2020) Assessment of groundwater quality status by using water quality index (WQI) and geographic information system (GIS) approaches: a case study of the Bokaro district, India. Appl Water Sci 10:1–16. https://doi.org/10.1007/s13201-019-1088-4
WHO (1996) Guidelines for drinking water quality, 2nd edn. World Health Organization, Geneva, Geneva
WHO (1997) GUIDELINES FOR DRINKING-WATER QUALITY. Geneva
WHO (2004) Guidelines for drinking water quality. In: Recommendations vol 1, 3rd edn Geneva
WHO (2009) Hardness in drinking-water background document for development of WHO guidelines for drinking-water quality
WHO (2011a) Guidelines for Drinking-water Quality. Geneva
WHO (2011b) Guidelines for drinking-water quality. World Health Organization, Geneva 27, Switzerland
WHO (2011c) Nitrate and nitrite in drinking-water
WHO (2017a) WHO - Guidelines on drinking water quality, 4th edn. World Health Organization, Geneva
WHO (2017b) Water quality and health - review of Turbidity: Information for regulators and water suppliers. 1–10
WRD (2007a) Status report on water quality of water bodies and ground water in Maharashtra for the year 2004–2005
WRD (2007b) Status report on water quality of water bodies and ground water in Maharashtra for the year 2004–2005. Nashik
Wagh VM, Panaskar DB, Mukate SV et al (2020) Nitrate associated health risks from groundwater of Kadava River Basin Nashik, Maharashtra, India. Hum Ecol Risk Assess an Int J 26:654–672. https://doi.org/10.1080/10807039.2018.1528861
Young WF, Horth H, Crane R et al (1996) Taste and odour threshold concentrations of potential potable water contaminants. Water Res 30:331–340. https://doi.org/10.1016/0043-1354(95)00173-5
Zoeteman BCJ (1980) Sensory assessment of water quality. Pergamon Press
Funding
This study was not funded by any agency/organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dandge, K.P., Patil, S.S. Spatial distribution of ground water quality index using remote sensing and GIS techniques. Appl Water Sci 12, 7 (2022). https://doi.org/10.1007/s13201-021-01546-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01546-7