Abstract
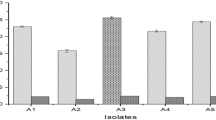
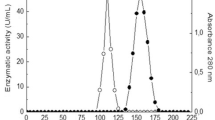
The use of better carbon sources and efficient production strains were deemed as promising strategies to economize tannase production. A novel agro-residue, cashew testa, was tested for the production of tannase under solid-state fermentation (SSF) using Aspergillus niger CEPC 11. CEPC 11 was identified by 18S rDNA typing as Aspergillus niger and deposited in International depositing Authority under MTCC number 5898 and NCBI accession number KM516789. The enzyme was purified 11 fold to obtain tannase with a specific activity of 10.22 U/mg and final yield of 48 %. SDS-PAGE analysis of purified enzyme gave a single band of 89.9KDa. The optimal temperature was found to be 40 °C, with an active range of 25–60 °C. The optimal pH was 5.5, and the enzyme was inactive at pH 8.0. The enzyme was identified through MALDI-TOF-MS tandem mass spectrometry as tannase. Km and Vmax were recorded at 0.1133 M (substrate concentration) and 44.79 μmol/min respectively. Heavy metals (Cadmium, Nickel, Lead, and Copper) in tannery effluent were analyzed before and after treatment with enzyme by AAS (Atomic Absorption spectroscopy). Gallic acid is also determined as an inter-mediatory by-product of this technology. Treatment with tannase enzyme improved the quality of fruit juices. This is the first report on production of tannase by Aspergillus niger under SSF with cashew industry by-product cashew testa as the substrate. The use of residues is an alternative to solve pollution problems that can be caused by an incorrect environmental disposal.





Similar content being viewed by others
References
Anwar YAS, Artika I (2007) The production of tannin acyl hydrolase from aspergillus niger. Microbiol Indones 1(2):9
Banerjee D, Mondal KC, Pati BR (2007) Tannase production by aspergillus aculeatus DBF9 through solid-state fermentation. Acta Microbiol Immunol Hung 54(2):159–166
Barthomeuf C, Regerat F, Pourrat H (1994) Production, purification and characterization of a tannase from aspergillus niger LCF 8. J Ferment Bioeng 77(3):320–323
Battestin V, Macedo GA (2007a) Tannase production by paecilomyces variotii. Bioresour Technol 98(9):1832–1837
Battestin V, Macedo GA (2007b) Effects of temperature, pH and additives on the activity of tannase produced by paecilomyces variotii. Electron J Biotechnol 10(2):191–199
Belmares R, Contreras-Esquivel JC, Rl R-H, Coronel AR, Aguilar CN (2004) Microbial production of tannase: an enzyme with potential use in food industry. LWT Food Sci Technol 37(8):857–864
Beniwal V, Kumar A, Sharma J, Chhokar V (2013) Recent advances in industrial application of tannases: a review. Recent Patents Biotechnol 7:228–233
Bhardwaj R, Singh B, Bhat TK (2003) Purification and characterization of tannin acyl hydrolase from aspergillus niger MTCC 2425. J Basic Microbiol 43(6):449–461
Bhat TK, Singh B, Sharma OP (1998) Microbial degradation of tannase-current perspective. Biodegradation 9(5):343–357
Harris JL (1986) Modified method for fungal slide culture. J Clin Microbiol 24(3):460–461
Haslam E, Stangroom JE (1966) The esterase and depsidase activities of tannase. Biochem J 99(1):28
Haworth RD, Jones K & Rogers HJ (1985) Ion-exchange chromatography of Aspergillus niger extract. Royal soc chemistry Thomas graham house, science park, Milton rd, Cambridge cb4 0wf, Cambs, England, p. 8–9
Iwamoto K, Tsuruta H, Nishitaini Y, Osawa R (2008) Identification and cloning of a gene encoding tannase (tannin acylhydrolase) from lactobacillus plantarum ATCC 14917 T. Syst Appl Microbiol 31(4):269–277
Jana A, Maity C, Halder SK, Das A, Pati BR, Mondal KC, Das Mohapatra PK (2013) Structural characterization of thermostable, solvent tolerant, cytosafe tannase from bacillus subtilis PAB2. Biochem Eng J 77:161–170
Kanagaraj J, Mandal AB (2012) Combined biodegradation and ozonation for removal of tannins and dyes for the reduction of pollution loads. Environ Sci Pollut Res 19(1):42–52
Kazemi M, Dakhili M, Dadkhah A, Yasarebifar Z, Larijani K (2011) Composition, antimicrobial and antioxidant activities of the essential oil of artemisia kermanesis podl, an endemic species from Iran. L J Med Plant Res 5(18):4481–4486
Klich MA and Pitt JI (1988) A laboratory guide to the common Aspergillus species and their teleomorphs. Commonwealth Scientific and Industrial Research Organization, Division of Food Processing. New South Wales, Australia, p 116
Kumar R, Sharma J, Singh R (2007) Production of tannase from aspergillus ruber under solid-state fermentation using jamun Syzygium cumini leaves. Microbiol Res 162(4):384–390
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lee SB (1990) Isolation of DNA from fungal mycelia and single spores. PCR protocols, a guide to methods and applications 282–287
Lekha PK, Lonsane BK (1997) Production and application of tannin acyl hydrolase: state of the art. Adv Appl Microbiol 44:216–260
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Marco-Gm M, Rodríguez LV, Ramos EL, Renovato J, Cruz-Hernández MA, Rodríguez R, Contreras J, Aguilar CN (2009) A novel tannase from the xerophilic fungus aspergillus niger GH1. J Microbiol Biotechnol 1:1–10
Mondal KC, Pati BR (2000) Studies on the extracellular tannase from newly isolated bacillus licheniformis KBR 6. J Basic Microbiol 40(4):223–232
Mukherjee G, Banerjee R (2004) Biosynthesis of tannase and gallic acid from tannin rich substrates by Rhizopus oryzae and aspergillus foetidus. J Basic Microbiol 44(1):42–48
O’Donnell K (1993) In: Reynolds DR, Taylor JW (eds) Fusarium and its near relatives in: the fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, UK, pp 225–233
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid state fermentation for the production of industrial enzymes. Curr Sci 77(1):149–162
Paranthaman R, Vidyalakshmi R, Murugesh S, Singaravadivel K (2008) Optimisation of fermentation conditions for production of tannase enzyme by aspergillus oryzae using sugarcane baggase and rice straw. Glob J Biotechnol Biochem 3:105–110
Paranthaman R, Vidyalakshmi R, Murugesh S, Singaravadivel K (2009) Optimization of various culture media for tannase production in submerged fermentation by aspergillus flavus. Adv Biol Res 3(1–2):34–39
Rajkumar GS, Nandy SC (1983) Isolation, purification and some properties of penicillium chrysogenium tannase. Appl Environ Microbiol 46:525–527
Ramirez-Coronel A, Darvill G, Viniegera-Gonzalez, Augur C (2003) Characterization of biofunctional tannase from aspergillus niger. Microbiology 149:2941–2946
Rout S, Banerjee R (2006) Production of tannase under mSSF and its application in fruit juice debittering. Indian J Biotechnol 5(3):346–350
Sabu A, Pandey A, Jaafar Daud M, Szakacs G (2005) Tamarind seed powder and palm kernel cake: two novel agro residues for the production of tannase under solid state fermentation by aspergillus niger ATCC 16620. Bioresour Technol 96(11):1223–1228
Sabu A, Augur C, Swati C, Pandey A (2006) Tannase production by lactobacillus sp. ASR-S1 under solid-state fermentation. Process Biochem 41(3):575–580
Seth M, Chand S (2000) Biosynthesis of tannase and hydrolysis of tannins to gallic acid by aspergillus awamori optimisation of process parameters. Process Biochem 36(1):39–44
Van de Lagemaat J, Pyle DL (2001) Solid-state fermentation and bioremediation: development of a continuous process for the production of fungal tannase. Chem Eng J 84(2):115–123
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viswanath, V., Leo, V.V., Prabha, S.S. et al. Biosynthesis of tannase from cashew testa using Aspergillus niger MTCC5889 by solid state fermentation. J Food Sci Technol 52, 7433–7440 (2015). https://doi.org/10.1007/s13197-015-1858-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1858-4