Abstract
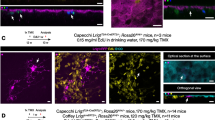
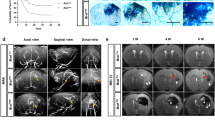
We have previously demonstrated that deletion of activin receptor-like kinase 1 (Alk1) or endoglin in a fraction of endothelial cells (ECs) induces brain arteriovenous malformations (bAVMs) in adult mice upon angiogenic stimulation. Here, we addressed three related questions: (1) could Alk1− mutant bone marrow (BM)-derived ECs (BMDECs) cause bAVMs? (2) is Alk1− ECs clonally expended during bAVM development? and (3) is the number of mutant ECs correlates to bAVM severity? For the first question, we transplanted BM from PdgfbiCreER;Alk12f/2f mice (EC-specific tamoxifen-inducible Cre with Alk1-floxed alleles) into wild-type mice, and then induced bAVMs by intra-brain injection of an adeno-associated viral vector expressing vascular endothelial growth factor and intra-peritoneal injection of tamoxifen. For the second question, clonal expansion was analyzed using PdgfbiCreER;Alk12f/2f;confetti+/− mice. For the third question, we titrated tamoxifen to limit Alk1 deletion and compared the severity of bAVM in mice treated with low and high tamoxifen doses. We found that wild-type mice with PdgfbiCreER;Alk12f/2f BM developed bAVMs upon VEGF stimulation and Alk1 gene deletion in BMDECs. We also observed clusters of ECs expressing the same confetti color within bAVMs and significant proliferation of Alk1− ECs at early stage of bAVM development, suggesting that Alk1− ECs clonally expanded by local proliferation. Tamoxifen dose titration revealed a direct correlation between the number of Alk1− ECs and the burden of dysplastic vessels in bAVMs. These results provide novel insights for the understanding of the mechanism by which a small fraction of Alk1 or endoglin mutant ECs contribute to development of bAVMs.






Similar content being viewed by others
Availability of Data and Material
The authors declare that all data supporting the findings of this study are available in the paper and its Supplementary Information.
Code Availability
Not applicable.
References
Gauden AJ, McRobb LS, Lee VS, Subramanian S, Moutrie V, Zhao Z, et al. Occlusion of animal model arteriovenous malformations using vascular targeting. Transl Stroke Res. 2020;11(4):689–99.
Pan P, Weinsheimer S, Cooke D, Winkler E, Abla A, Kim H, et al. Review of treatment and therapeutic targets in brain arteriovenous malformation. J Cereb Blood Flow Metab. 2021:271678X211026771. https://doi.org/10.1177/0271678X211026771
Bharatha A, Faughnan ME, Kim H, Pourmohamad T, Krings T, Bayrak-Toydemir P, et al. Brain arteriovenous malformation multiplicity predicts the diagnosis of hereditary hemorrhagic telangiectasia: quantitative assessment. Stroke. 2012;43(1):72–8.
Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl. 2011;111:83–92.
Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69(6):954–62.
Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One. 2014;9(2):e88511.
Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, et al. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke. 2014;45(3):900–2.
Zhu W, Saw D, Weiss M, Sun Z, Wei M, Shaligram S, et al. Induction of brain arteriovenous malformation through CRISPR/Cas9-mediated somatic Alk1 gene mutations in adult mice. Transl Stroke Res. 2019;10(5):557–65.
Chen W, Choi EJ, McDougall CM, Su H. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl Stroke Res. 2014;5(3):316–29.
Garrido-Martin EM, Nguyen HL, Cunningham TA, Choe SW, Jiang Z, Arthur HM, et al. Common and distinctive pathogenetic features of arteriovenous malformations in hereditary hemorrhagic telangiectasia 1 and hereditary hemorrhagic telangiectasia 2 animal models–brief report. Arterioscler Thromb Vasc Biol. 2014;34(10):2232–6.
Choi EJ, Walker EJ, Shen F, Oh SP, Arthur HM, Young WL, et al. Minimal homozygous endothelial deletion of Eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc Dis. 2012;33(6):540–7.
Hao Q, Liu J, Pappu R, Su H, Rola R, Gabriel RA, et al. Contribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leukocytes and macrophages. Arterioscler Thromb Vasc Biol. 2008;28(12):2151–7.
Choi EJ, Walker EJ, Degos V, Jun K, Kuo R, Pile-Spellman J, et al. Endoglin deficiency in bone marrow is sufficient to cause cerebrovascular dysplasia in the adult mouse after vascular endothelial growth factor stimulation. Stroke. 2013;44(3):795–8.
Jin Y, Muhl L, Burmakin M, Wang Y, Duchez AC, Betsholtz C, et al. Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nat Cell Biol. 2017;19(6):639–52.
Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450(7166):56–62.
Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337(6095):730–5.
Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, et al. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2 (HHT2). Blood. 2008;111(2):633–42.
Pontes-Quero S, Heredia L, Casquero-Garcia V, Fernandez-Chacon M, Luo W, Hermoso A, et al. Dual ifgMosaic: a versatile method for multispectral and combinatorial mosaic gene-function analysis. Cell. 2017;170(4):800-14e18.
Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, et al. Activin receptor-like kinase 1 modulates transforming growth factor- beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97(6):2626–31.
Corti P, Young S, Chen CY, Patrick MJ, Rochon ER, Pekkan K, et al. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development. 2011;138(8):1573–82.
Chen Y, Ma L, Yang S, Burkhardt JK, Lu J, Ye X, et al. Quantitative angiographic hemodynamic evaluation after revascularization surgery for moyamoya disease. Transl Stroke Res. 2020;11(5):871–81.
Kim YH, Phuong NV, Choe SW, Jeon CJ, Arthur HM, Vary CP, et al. Overexpression of activin receptor-like kinase 1 in endothelial cells suppresses development of arteriovenous malformations in mouse models of hereditary hemorrhagic telangiectasia. Circ Res. 2020;127(9):112–1137.
Acknowledgements
We thank Dr. Ludmila Pawlikowska for assistance with manuscript preparation.
Funding
This study was supported by the National Institutes of Health (R01 HL122774, NS027713 and NS112819) and from the Michael Ryan Zodda Foundation to H.S.; AHA fellowship (20POST35120371) to N.S.; and the US Department of Defense (PR161205), the Leducq Foundation (ATTRACT), and Barrow Neurological Foundation to S.P.O.
Author information
Authors and Affiliations
Contributions
HS, SS, and TA designed the experiment. SS, ML, QL, RZ, LM, MW, SNG, RL, LP, CT, and FP collected and analyzed the data. SS and HS drafted the manuscript. TA, PP, SPO, and HS critically read the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The protocol and experimental procedures for using laboratory animals were approved by the Institution of Animal Care and Use Committee (IACUC) at the University of California, San Francisco.
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shaligram, S.S., Zhang, R., Zhu, W. et al. Bone Marrow-Derived Alk1 Mutant Endothelial Cells and Clonally Expanded Somatic Alk1 Mutant Endothelial Cells Contribute to the Development of Brain Arteriovenous Malformations in Mice. Transl. Stroke Res. 13, 494–504 (2022). https://doi.org/10.1007/s12975-021-00955-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-021-00955-9