Abstract
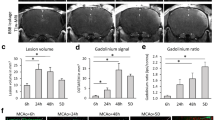
Recombinant T cell Receptor Ligand 1000 (RTL1000), a partial human major histocompatibility complex (MHC) molecule coupled to a human myelin peptide, reduces infarct size after experimental stroke in HLA-DRB1*1502 transgenic (DR2-Tg) mice. In this study, we characterized the therapeutic time window of opportunity for RTL1000; we explored the efficacy of a single dose of RTL1000 administration and determined if RTL1000 affords long-term neurobehavioral functional improvement after ischemic stroke. Male DR2-Tg mice underwent 60 min of intraluminal reversible middle cerebral artery occlusion (MCAO). RTL1000 or vehicle was injected 4, 6, or 8 h after MCAO, followed by three daily injections. In the single-dose study, one-time injection of RTL1000 was applied 4 h after MCAO. Cortical, striatal, and hemispheric infarct sizes were measured 24 or 96 h after stroke. Behavioral testing, including neuroscore evaluation, open field, paw preference, and novel object recognition, was performed up to 28 days after stroke. Our data showed that RTL1000 significantly reduced the infarct size 96 h after MCAO when the first injection was given at 4 and 6 h, but not 8 h, after the onset of stroke. A single dose of 400 or 100 μg RTL1000 also significantly reduced the infarct size 24 h after MCAO. Behavioral testing showed that RTL1000 treatment used 4 h after MCAO improved long-term cognitive outcome 28 days after stroke. Taken together, RTL1000 protects against acute injury if applied within a 6-h time window and improves long-term functional recovery after experimental stroke in DR2-Tg mice.





Similar content being viewed by others
References
Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–3.
Muir KW, Tyrrell P, Sattar N, et al. Inflammation and ischemic stroke. Curr Opin Neurol. 2007;20:334–42.
Gee JM, Kalil A, Shea C, et al. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38(2 Suppl):783–8.
NilupulPerera M, Ma HK, Arakawa S, et al. Inflammation following stroke. J Clin Neurosci. 2006;13(1):1–8.
Hurn PD, Subramanian S, Parker SM, et al. T- and B-cell deficient mice with experimental stroke has reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–805.
Yilmza G, Arumugan TV, Stokes KY, et al. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–12.
Offner H, Subramanian S, Parker SM, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–65.
Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–111.
Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–31.
Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is medicated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–36.
Burrows GG, Chang JW, Bachinger HP, et al. Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12:771–8.
Wang C, Mooney JL, Meza-Romero R, et al. Recombinant TCR ligand induces early TCT signaling and a unique pattern of downstream activation. J Immunol. 2003;171:1934–40.
Vandenbark AA, Rich C, Mooney J, et al. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35–55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in DR2-TG transgenic mice. J Immunol. 2003;171:127–33.
Subramanian S, Zhang B, Kosaka Y, et al. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40:2539–45.
Dziennis S, Mader S, Akiyoshi K, et al. Therapy with recombinant T-cell receptor ligand reduces infarct size and infiltrating inflammatory cells in brain after middle cerebral artery occlusion in mice. Metab Brain Dis. 2011;26:123–33.
Yadav V, Dennis NB, Bowen JD, et al. Recombinant T-cell receptor ligand (RTL) for treatment of multiple sclerosis: a double-blind, placebo-controlled, phase I, dose-escalation study. Autoimmune Dis. 2012. doi:10.1155/2012/954739. Article ID: 954739.
Fisher M, Feuerstein G, David WH, et al. Update of stroke therapy academic industry rountable preclinical recommendations. Stroke. 2009;40:2244–50.
Stroke Therapy Academic Industry Roundtable. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–58.
Gonzalez-Gay MA, Zanelli E, Khare SD, et al. Human leukocyte antigen-DRB1*1502 (DR2-TGDW12) transgene reduces incidence and severity of arthritis in mice. Hum Immunol. 1996;50:54–60.
Huan JY, Meza-Romero R, Mooney JL, et al. Rationally designed mutations convert complexes of human recombinant T cell receptor ligands into monomers that retain biological activity. J Chem Technol Biotechnol. 2005;80:2–12.
Zhu W, Wang L, Zhang L, et al. Isoflurane preconditioning neuroprotection in experimental focal stroke is androgen-dependent in male mice. Neuroscience. 2010;169:758–69.
Zhang W, Davis CM, Edin ML, et al. Role of endothelial soluble epoxide hydrolase in cerebrovascular function and ischemic injury. PLoS One. 2013;8(4):e61244.
Uchida M, Palmateer JM, Herson PS, et al. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–62.
Schallert T, Leasure JL, Kolb B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab. 2000;20:1513–28.
Craft TK, Zhang N, Glasper ER, et al. Neonatal factors influence adult stroke outcome. Psychoneuroendocrinology. 2006;31:601–13.
Li X, Blizzard KK, Zhu Z, et al. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104.
Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34.
Akiyoshi K, Dziennis S, Palmateer J, et al. T cell receptor ligands improve outcome after experimental cerebral ischemia. Trans Stroke Res. 2011;2:404–10.
Vandenbark AA, Meza-Romero R, Benedek G, et al. A novel regulatory pathway for autoimmune disease: binding of partial MHC class II constructs to monocytes reduces CD74 expression and induces both specific and bystander T-cell tolerance. J Autoimm. 2013;40C:96–110.
Ren X, Akiyoshi K, Grafe MR, et al. Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. Metab Brain Dis. 2012;27:7–15.
Benedek G, Meza-Romero R, Andrew S, et al. Partial MHC class II constructs inhibit MIF/CD74 binding and downstream effects. Eur J Immunol. 2013;43:1309–21.
Wang C, Gold BG, Kaler LJ, et al. Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J Neurochem. 2006;98:1817–27.
Acknowledgments
This work was supported by NIH Grants #NS076013 (STTR) and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
Conflict of Interest
Dr. Offner, Dr. Alkayed and OHSU have a significant financial interest in ArtielleImmuno Therapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAMC Conflict of Interest in Research Committees. Wenbin Zhu declares that he has no conflict of interest. Amanda Casper declares that she has no conflict of interest. Nicole L. Libal declares that she has no conflict of interest. Stephanie J. Murphy declares that she has no conflict of interest. Sheetal Bodhankar declares that she has no conflict of interest. All institutional and national guidelines for the care and use of laboratory animals were followed. This article does not contain any studies with human subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, W., Casper, A., Libal, N.L. et al. Preclinical Evaluation of Recombinant T Cell Receptor Ligand RTL1000 as a Therapeutic Agent in Ischemic Stroke. Transl. Stroke Res. 6, 60–68 (2015). https://doi.org/10.1007/s12975-014-0373-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-014-0373-7