Abstract
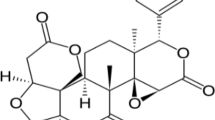
The present study involved to synthesize reduced grapheme oxide (rGO) nanocomposite–decorated lotus flower–like selenium dioxide (SeO2) nanomaterial by the sonochemical method, and it was utilized for electrochemical detection of quercetin (QR) (a flavonoid) in natural food sample, urine, and blood serum samples. The as-synthesized nanomaterial was characterized by XRD, FT-IR, Raman, BET, FE-SEM, EDX, and mapping analysis techniques. The SeO2/rGO nanocomposite modified on glassy carbon electrode (GCE) showed an excellent electrocatalytic activity towards the detection of QR as compared with other modified GCEs, and bare GCE. Moreover, the present electrochemical sensor was exhibited a superior current response for sensing of QR with wide linear ranges (from 0.01 to 200 µM), high sensitivity (71.023 µA µM−1 cm−2), and a lowest limit of detection (LOD) of 0.0016 µM. Besides, the fabricated SeO2/rGO nanocomposite–modified GCE was provided an excellent selectivity for QR in the presence of facile biological and inorganic interfering species. The fabricated SeO2/rGO-modified GCE was utilized for the determination of QR in various real samples, and the obtained data showed good recovery results.
Graphical Abstract








Similar content being viewed by others
References
X. Lou, B. Yuan, L. Wang, H. Xu, M. Hanna, L. Yuan, Evaluation of physicochemical characteristics, nutritional composition and antioxidant capacity of Chinese organic hawthorn berry (Crataegus pinnatifida). Int. J. Food Sci. Technol. 55, 1679–1688 (2020). https://doi.org/10.1111/ijfs.14437
D. Oliveira, C. Latimer, P. Parpot, C.I.R. Gill, R. Oliveira, Antioxidant and antigenotoxic activities of Ginkgo biloba L. leaf extract are retained after in vitro gastrointestinal digestive conditions, Eur. J. Nutr. 59, 465–476 (2020). https://doi.org/10.1007/s00394-019-01915-8
X. Zhang, J. Huang, C. Yu, L. Xiang, L. Li, D. Shi, F. Lin, Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ros production. Onco. Targets. Ther. 13, 513–523 (2020). https://doi.org/10.2147/OTT.S228453
G.K. Ziyatdinova, S.P. Zakharova, E.R. Ziganshina, H.C. Budnikov, Voltammetric determination of flavonoids in medicinal plant materials using electrodes modified by cerium dioxide nanoparticles and surfactants. J. Anal. Chem. 74, 816–824 (2019). https://doi.org/10.1134/S106193481908015X
W. Huang, K. Seehafer, U.H.F. Bunz, Discrimination of flavonoids by a hypothesis free Sensor array. ACS Appl. Polym. Mater. 1, 1301–1307 (2019). https://doi.org/10.1021/acsapm.9b00116
N. Sebastian, W.C. Yu, D. Balram, Synthesis of amine-functionalized multi-walled carbon nanotube/3D rose flower-like zinc oxide nanocomposite for sensitive electrochemical detection of flavonoid morin. Anal. Chim. Acta. 1095, 71–81 (2020). https://doi.org/10.1016/j.aca.2019.10.026
J. Hu, Z. Zhang, Application of electrochemical sensors based on carbon nanomaterials for detection of flavonoids. Nanomaterials 10, 1–14 (2020). https://doi.org/10.3390/nano10102020
H. Li, S. Wang, F. Cui, B. Zhuo, C. Zhao, W. Liu, Sensitive and selective detection of puerarin based on the hybrid of reduced graphene oxide and molecularly imprinted polymer. J. Pharm. Biomed. Anal. 185, 113221 (2020). https://doi.org/10.1016/j.jpba.2020.113221
S. Tajik, Z. Dourandish, K. Zhang, H. Beitollahi, Q.V. Le, H.W. Jang, M. Shokouhimehr, Carbon and graphene quantum dots: a review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC. Adv. 10, 15406–15429 (2020). https://doi.org/10.1039/D0RA00799D
Z. Zhou, P. Zhao, C. Wang, P. Yang, Y. Xie, J. Fei, Ultra-sensitive amperometric determination of quercetin by using a glassy carbon electrode modified with a nanocomposite prepared from aminated graphene quantum dots, thiolated β-cyclodextrin and gold nanoparticles. Microchim. Acta. 187, 1–9 (2020). https://doi.org/10.1007/s00604-019-4106-1
S. Feng, J. Guo, X. Chen, J. Meng, G. Zhang, G. Liu, H. Sun, B. Wang, W. Liu, N, S co-doped graphene/Ag@Au triangular core-shell nanomaterials for determination of quercetin, Int. J. Electrochem. Sci. 15, 8041–8054 (2020). https://doi.org/10.20964/2020.08.44
X. Zhang, B. Shao, L. Yan, Y. Lu, M. Zhao, X. He, X. Li, W. Sun, Pyronine B/graphene copolymer modified carbon molecular wire electrode for electrochemical Detection of Quercetin, Int. J. Electrochem. Sci. 16, 150960 (2021). https://doi.org/10.20964/2021.01.66
V. Vinothkumar, A. Sangili, S.M. Chen, P. Veerakumar, K.C. Lin, Sr-Doped NiO3 nanorods synthesized by a simple sonochemical method as excellent materials for voltammetric determination of quercetin. New J. Chem. 44, 2821–2832 (2020). https://doi.org/10.1039/c9nj05660b
E. Kuyumcu Savan, Square Wave Voltammetric (SWV) Determination of quercetin in tea samples at a single-walled carbon nanotube (SWCNT) modified glassy carbon electrode (GCE), Anal. Lett. 53, 858–872 (2020). https://doi.org/10.1080/00032719.2019.1684514
G. Ran, Y. Li, Y. Xia, Graphene oxide and electropolymerized p-aminobenzenesulfonic acid mixed film used as dopamine and serotonin electrochemical sensor. Monatshefte Fur Chemie. 151, 293–299 (2020). https://doi.org/10.1007/s00706-020-02559-9
E. Demir, A. Senocak, M.F. Tassembedo-Koubangoye, E. Demirbas, H.Y. Aboul-Eneın, Electrochemical evaluation of the total antioxidant capacity of yam food samples on a polyglycine-glassy carbon modified electrode. Curr. Anal. Chem. 16, 176–183 (2020). https://doi.org/10.2174/1573411014666180619143729
J. Jing, Y. Shi, Q. Zhang, J. Wang, J. Ruan, Prediction of Chinese green tea ranking by metabolite profiling using ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry (UPLC–QTOF/MS). Food Chem. 221, 311–316 (2017). https://doi.org/10.1016/j.foodchem.2016.10.068
R. Ravichandran, M. Rajendran, D. Devapiriam, Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 146, 472–478 (2014). https://doi.org/10.1016/j.foodchem.2013.09.080
V. Pilařová, K. Plachká, L. Chrenková, I. Najmanová, P. Mladěnka, F. Švec, O. Novák, L. Nováková, Simultaneous determination of quercetin and its metabolites in rat plasma by using ultra-high performance liquid chromatography tandem mass spectrometry. Talanta 185, 71–79 (2018). https://doi.org/10.1016/j.talanta.2018.03.033
X. Lu, C.F. Ross, J.R. Powers, B.A. Rasco, Determination of quercetins in onion (Allium cepa) using infrared spectroscopy. J. Agric. Food Chem. 59, 6376–6382 (2011). https://doi.org/10.1021/jf200953z
R. Bugianesi, M. Serafini, F. Simone, D. Wu, S. Meydani, A. Ferro-Luzzi, E. Azzini, G. Maiani, Highperformance liquid chromatography with coulometric electrode array detector for the determination of quercetin levels in cells of the immune system. Anal. Biochem. 284, 296–300 (2000). https://doi.org/10.1006/abio.2000.4697
H. Duan, Y. Chen, G. Chen, Far infrared-assisted extraction followed by capillary electrophoresis for the determination of bioactive constituents in the leaves of Lycium barbarum Linn., J. Chromatogr. A. 1217, 4511–4516 (2010). https://doi.org/10.1016/j.chroma.2010.04.069
G. Chen, H. Zhang, J. Ye, Determination of rutin and quercetin in plants by capillary electrophoresis with electrochemical detection. Anal. Chim. Acta. 423, 69–76 (2000). https://doi.org/10.1016/S0003-2670(00)01099-0
D. Wu, Z. Chen, ZnS quantum dots-based fluorescence spectroscopic technique for the detection of quercetin. Luminescence 29, 307–313 (2014). https://doi.org/10.1002/bio.2545
J. Yu, H. Jin, R. Gui, W. Lv, Z. Wang, A facile strategy for ratiometric electrochemical sensing of quercetin in electrolyte solution directly using bare glassy carbon electrode. J. Electroanal. Chem. 795, 97–102 (2017). https://doi.org/10.1016/j.jelechem.2017.04.053
P. Zuo, D. Xiao, M. Gao, J. Peng, R. Pan, Y. Xia, H. He, Single-step preparation of fluorescent carbon nanoparticles, and their application as a fluorometric probe for quercetin. Microchim. Acta. 181, 1309–1316 (2014). https://doi.org/10.1007/s00604-014-1236-3
Z. Cai, H. Li, J. Wu, L. Zhu, X. Ma, C. Zhang, Ascorbic acid stabilised copper nanoclusters as fluorescent sensors for detection of quercetin. RSC Adv. 10, 8989–8993 (2020). https://doi.org/10.1039/d0ra01265c
A.N. Raja, Annu, R.Jain, Development of Aloe-vera-titanium oxide –based ultrasensitive sensor for the quantification of quercetin, Anal Sci Adv. 1, 56–59 (2020). https://doi.org/10.1002/ansa.202000010
Y. Ji, Y. Li, B. Ren, X. Liu, Y. Li, J. Soar, Nitrogen-doped graphene-ionic liquid-glassy carbon microsphere paste electrode for ultra-sensitive determination of quercetin. Microchem. J. 155, 104689 (2020). https://doi.org/10.1016/j.microc.2020.104689
Y. Peng, M. Liao, X. Ma, H. Deng, F. Gao, R. Dai, L. Lu, Electrochemical determination of rutin using SnO2/nitrogen-doped graphene composite electrode, Int. J. Electrochem. Sci. 14, 4946–4956 (2019). https://doi.org/10.20964/2019.06.28
A. Şenocak, Simple and sensitive detection of quercetin antioxidant by teos coated magnetic Fe2O3 core-shell, J. Turkish Chem. Soc. Sect. A Chem. 7, 525–534 (2020). https://doi.org/10.18596/jotcsa.733141
G. Ziyatdinova, E. Kozlova, H. Budnikov, Poly(gallic acid)/MWNT-modified electrode for the selective and sensitive voltammetric determination of quercetin in medicinal herbs. J. Electroanal. Chem. 821, 73–81 (2018). https://doi.org/10.1016/j.jelechem.2017.12.071
X. Niu, X. Li, W. Chen, X. Li, W. Weng, C. Yin, R. Dong, W. Sun, G. Li, Three-dimensional reduced graphene oxide aerogel modified electrode for the sensitive quercetin sensing and its application. Mater. Sci. Eng. C. 89, 230–236 (2018). https://doi.org/10.1016/j.msec.2018.04.015
J. Manokaran, R. Muruganantham, A. Muthukrishnaraj, N. Balasubramanan, Platinum –polydopamine@SiO2 nanocomposite modified electrode for the electrochemical determination of Quercetin. Electrochim Acta. 168, 16–24 (2015). https://doi.org/10.1016/j.electacta.2015.04.016
S. Tajyani, A. Babaei, A new sensing platform based on magnetic Fe3O4@NiO core/shell nanoparticles for simultaneous voltammetric determination of quercetin and tryptophan. J Electroanal Chem. 808, 50–58 (2018). https://doi.org/10.1016/j.jelechem.2017.11.010
M. Wang, D. Zhang, Z. Tong, X. Xu, X. Yang, Voltammetric behavior and the determination of quercetin at a flowerlike Co3O4 nanoparticles modified glassy carbon electrode. J. Appl. Electrochem. 41, 189–196 (2011). https://doi.org/10.1007/s10800-010-0223-6
A. Karthika, V. Ramasamy Raja, P. Karuppasamy, A. Suganthi, M. Rajarajan, A novel electrochemical sensor for determination of hydroquinone in water using FeWO4/SnO2 nanocomposite immobilized modified glassy carbon electrode, Arab. J. Chem. 13, 4065–4081 (2020). https://doi.org/10.1016/j.arabjc.2019.06.008
A. Karthika, A. Suganthi, M. Rajarajan, An in-situ synthesis of novel V2O5/G-C3N4/PVA nanocomposite for enhanced electrocatalytic activity toward sensitive and selective sensing of folic acid in natural samples. Arab. J. Chem. 13, 3639–3652 (2020). https://doi.org/10.1016/j.arabjc.2019.12.009
A. Karthika, D.R. Rosaline, S.S.R. Inbanathan, A. Suganthi, M. Rajarajan, Fabrication of Cupric oxidedecorated β-cyclodextrin nanocomposite solubilized Nafion as a high performance electrochemical sensor for L-tyrosine detection. J. Phys. Chem. Solids. 136, 109145 (2020). https://doi.org/10.1016/j.jpcs.2019.109145
A. Karthika, V. Ramasamy Raja, P. Karuppasamy, A. Suganthi, M. Rajarajan, Electrochemical behaviour and voltammetric determination of mercury (II) ion in cupric oxide/poly vinyl alcohol nanocomposite modified glassy carbon electrode, Microchem. J. 145, 737–744 (2019). https://doi.org/10.1016/j.microc.2018.11.030
X. Tian, L. Liu, Y. Li, C. Yang, Z. Zhou, Y. Nie, Y. Wang, Nonenzymatic electrochemical sensor based on CuO-TiO2 for sensitive and selective detection of methyl parathion pesticide in ground water. Sensors Actuators, B Chem. 256, 135–142 (2018). https://doi.org/10.1016/j.snb.2017.10.066
M. Siva Prasad, R. Chen, H. Ni, K. Kiran Kumar, Directly grown of 3D-nickel oxide nano flowers on TiO2 nanowire arrays by hydrothermal route for electrochemical determination of naringenin flavonoid in vegetable samples, Arab. J. Chem. 13, 1520–1531 (2020). https://doi.org/10.1016/j.arabjc.2017.12.004
A. Karthika, P. Karuppasamy, S. Selvarajan, A. Suganthi, M. Rajarajan, Electrochemical sensing of nicotine using CuWO4 decorated reduced graphene oxide immobilized glassy carbon electrode. Ultrason. Sonochem. 55, 196–206 (2019). https://doi.org/10.1016/j.ultsonch.2019.01.038
S. Chaudhary, S.K. Mehta, Selenium nanomaterials: applications in electronics, catalysis and sensors. J. Nanosci. Nanotechnol. 14, 1658–1674 (2014). https://doi.org/10.1166/jnn.2014.9128
D.M. Stanković, E. Mehmeti, J. Zavašnik, K. Kalcher, Determination of nitrite in tap water: a comparative study between cerium, titanium and selenium dioxide doped reduced graphene oxide modified glassy carbon electrodes. Sensors Actuators, B Chem. 236, 311–317 (2016). https://doi.org/10.1016/j.snb.2016.06.018
A.H. Shar, M.N. Lakhan, J. Wang, M. Ahmed, K.T. Alali, R. Ahmed, I. Ali, A.Q. Dayo, Facile synthesis and characterization of selenium nanoparticles by the hydrothermal approach. Dig. J. Nanomater. Biostructures. 14, 867–872 (2019)
S. Swathi, B.J. Rani, G. Ravi, R. Yuvakkumar, S.I. Hong, D. Velauthapillai, B. Saravanakumar, M. Thambidurai, C. Dang, Designing rational and cheapest SeO2 electrocatalyst for long stable water splitting process. J. Phys. Chem. Solids. 145, 109544 (2020). https://doi.org/10.1016/j.jpcs.2020.109544
G. Singh, A. Kushwaha, M. Sharma, Intriguing peroxidase-mimic for H2O2 and glucose sensing: A synergistic Ce2(MoO4)3/rGO nanocomposites, J. Alloys Compd. 825, 154134 (2020)
M. Dong, H. Hu, S. Ding, C. Wang, L. Li, Fabrication of NiMn2O4 nanosheets on reduced graphene oxide for non-enzymatic detection of glucose. Mater. Technol. (2020). https://doi.org/10.1080/10667857.2020.1740861
K. Sakthivel, G. Mani, S.M. Chen, S.H. Lin, A. Muthumariappan, V. Mani, A novel synthesis of nonaggregated spinel nickel ferrite nanosheets for developing non-enzymatic reactive oxygen species sensor in biological samples. J. Electroanal. Chem. 820, 161–167 (2018). https://doi.org/10.1016/j.jelechem.2018.04.058
M. Kazemi, A. Akbari, H. Zarrinfar, S. Soleimanpour, Z. Sabouri, M. Khatami, M. Darroudi, Evaluation of antifungal and photocatalytic activities of gelatin-stabilized selenium oxide nanoparticles. J Inorg Organomet Polym. 30, 3036–3044 (2020). https://doi.org/10.1007/s10904-020-01462-4
C. Sabarinathan, P. Karuppasamy, C.T. Vijayakumar, T. Arumuganathan, Development of methylene blue removal methodology by adsorption using molecular polyoxometalate: Kinetics, Thermodynamics and Mechanistic Study. Microchem. J. 146, 315–326 (2019). https://doi.org/10.1016/j.microc.2019.01.015
K.J. Huang, L. Wang, J. Li, M. Yu, Y.M. Liu, Electrochemical sensing of catechol using a glassy carbon electrode modified with a composite made from silver nanoparticles, polydopamine, and graphene. Microchim. Acta. 180, 751–757 (2013). https://doi.org/10.1007/s00604-013-0988-5
A.R. Rajamani, S.C. Peter, Novel nanostructured Pt/CeO2@Cu2O carbon-based electrode to magnify the electrochemical detection of the neurotransmitter dopamine and analgesic paracetamol. ACS Appl. Nano Mater. 1, 5148–5157 (2018). https://doi.org/10.1021/acsanm.8b01217
A.T.E. Vilian, P. Puthiaraj, C.H. Kwak, S.R. Choe, Y.S. Huh, W.S. Ahn, Y.K. Han, Electrochemical determination of quercetin based on porous aromatic frameworks supported Au nanoparticles. Electrochim. Acta. 216, 181–187 (2016). https://doi.org/10.1016/j.electacta.2016.08.150
J. Rembiesa, H. Gari, J. Engblom, T. Ruzgas, Amperometric monitoring of quercetin permeation through skin membranes. Int. J. Pharm. 496, 636–643 (2015). https://doi.org/10.1016/j.ijpharm.2015.10.073
S.K. Ponnaiah, P. Periakaruppan, A glassy carbon electrode modified with a copper tungstate and polyaniline nanocomposite for voltammetric determination of quercetin. Microchim. Acta. 185, 1–7 (2018). https://doi.org/10.1007/s00604-018-3071-4
Z. Liang, H. Zhai, Z. Chen, S. Wang, H. Wang, S. Wang, A sensitive electrochemical sensor for flavonoids based on a multi-walled carbon paste electrode modified by cetyltrimethyl ammonium bromide-carboxylic multi-walled carbon nanotubes. Sensors Actuators, B Chem. 244, 897–906 (2017). https://doi.org/10.1016/j.snb.2016.12.108
M. Mosleh, S.M. Ghoreishi, S. Masoum, A. Khoobi, Determination of quercetin in the presence of tannic acid in soft drinks based on carbon nanotubes modified electrode using chemometric approaches. Sensors Actuators, B Chem. 272, 605–611 (2018). https://doi.org/10.1016/j.snb.2018.05.172
S. Sun, M. Zhang, Y. Li, X. He, A molecularly imprinted polymer with incorporated Graphene oxide for electrochemical determination of quercetin. Sensors (Switzerland). 13, 5493–5506 (2013). https://doi.org/10.3390/s130505493
J. Li, J. Qu, R. Yang, L. Qu, P.B. Harrington, A sensitive electrochemical sensor of Quercetin based on graphene quantum dots/gold nanoparticles nanocomposite. Electroanal. 28, 1322–1330 (2016). https://doi.org/10.1002/elan.201500490
J.V. Piovesan, A. Spinelli, Determination of quercetin in a pharmaceutical sample by square-wave voltammetry using a poly(vinylpyrrolidone)-modified carbon-paste electrode. In: J. Braz. Chem. Soc. Sociedade Brasileira de Quimica, pp. 517–525 (2014). https://doi.org/10.5935/0103-5053.20140019
S. Muthamizh, R. Suresh, K. Giribabu, R. Manigandan, S. Praveen Kumar, S. Munusamy, V. Narayanan, MnWO4 nanocapsules: synthesis, characterization and its electrochemical sensing property. J. Alloys Compd. 619, 601–609 (2015). https://doi.org/10.1016/j.jallcom.2014.09.049
M. Veerapandian, Y.T. Seo, K. Yun, M.H. Lee, Graphene oxide functionalized with silver@silicapolyethylene glycol hybrid nanoparticles for direct electrochemical detection of quercetin. Biosens. Bioelectron. 58, 200–204 (2014). https://doi.org/10.1016/j.bios.2014.02.062
M.L. Yola, N. Atar, Z. Üstündaǧ, A.O. Solak, A novel voltammetric sensor based on p-aminothiophenol functionalized graphene oxide/gold nanoparticles for determining quercetin in the presence of ascorbic acid. J. Electroanal. Chem. 698, 9–16 (2013). https://doi.org/10.1016/j.jelechem.2013.03.016
S. Fei, J. Chen, S. Yao, G. Deng, D. He, Y. Kuang, Electrochemical behavior of L-cysteine and its detection at carbon nanotube electrode modified with platinum. Anal. Biochem. 339, 29–35 (2005). https://doi.org/10.1016/j.ab.2005.01.002
K. Venkatesh, B. Muthukutty, S.M. Chen, C. Karuppiah, B. Amanulla, C.C. Yang, R. Sayee Kannan, Nanomolar level detection of non-steroidal antiandrogen drug flutamide based on ZnMn2O4 nanoparticles decorated porous reduced graphene oxide nanocomposite electrode. J. Hazard. Mater. 405, 124096 (2021). https://doi.org/10.1016/j.jhazmat.2020.124096
Acknowledgements
The author thanks the Post Graduate and Research Department of Chemistry, Post graduate and Research Department of Chemistry, Vivekananda College, Madurai 625234, Tamil Nadu, India, for providing laboratory facilities to carry out the present work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
All authors have participated in the conception, design, analysis, and interpretation of the data; drafting and revision of the article; and approval of the final version. This manuscript has not been submitted to any other journal or under review. The authors have not used any financial assistance or fund to carry out this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karuppasamy, P., Karthika, A., Senthilkumar, S. et al. An Efficient and Highly Sensitive Amperometric Quercetin Sensor Based on a Lotus Flower Like SeO2-Decorated rGO Nanocomposite Modified Glassy Carbon Electrode. Electrocatalysis 13, 269–282 (2022). https://doi.org/10.1007/s12678-022-00707-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-022-00707-9