Abstract
Exosomes can be released by a variety of cells and participate in intercellular communication in many physiological processes in the body. They can be used as carriers of cancer therapeutic drugs and have natural delivery capabilities. Some biologically active substances on exosomes, such as major histocompatibility complex (MHC), have been shown to be involved in exosome-mediated anticancer immune responses and have important regulatory effects on the immune system. Exosome-based drug delivery systems hold great promise in future cancer immunotherapy. However, there are still substantial challenges to be overcome in the clinical application of exosomes as drug carriers. This article reviews the biological characteristics of exosome drug delivery systems and their potential applications and challenges in cancer immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The term “exosomes” first appeared in the 1980s. Trams et al. discovered a set of vesicle-like structures with diameters ranging from 40 to 1000 nm using transmission electron microscopy [1]. Later, Johnstone isolated these vesicles from sheep reticulocytes by ultracentrifugation at 100,000×g for 90 min, and these vesicle-like structures were called exosomes for the first time [2].
The extracellular vesicles (EVs) collectively refers to various vesicles with membrane structures released by cells. Due to their different sizes and how they are formed, they are divided into three subgroups: exosomes, microvesicles, and apoptotic bodies (Table 1). Exosomes, also known as intraluminal vesicles (ILVs), are approximately 40–160 nm in size and are produced by inward budding of multivesicular endosomes (MVEs) during maturation [3, 4]. Cells release exosomes after MVEs fuse with the cell membrane. The endosomal sorting complex (ESCRT), Rab protein, CD36 and sphingolipid ceramide required for the transport mechanism have been shown to play important roles in biological processes [5,6,7,8]. Exosomes can be secreted under physiological and pathological conditions by almost all types of cells, including prokaryotic cells and eukaryotic cells [9, 10]. They are widely present in culture supernatants and biological fluids such as blood, urine, breast milk, pleural fluid and cerebrospinal fluid. The exosomes released from one cell type (donor cells) can be taken up by another (recipient cells). If release and uptake of exosomes are arisen by same cells, it is called to be autologous (or autocrine). If these actions are achieved between different or remote cell types, it is called to be heterologous (or paracrine) [8, 11].
In recent years, the concept of precision medicine has been widely accepted. To improve the therapeutic effect of drugs and reduce toxicity and side effects, researchers have gradually begun to pay attention to precise and efficient drug delivery systems. Exosomes have been found to be good carriers for drug delivery systems [12,13,14]. They can transport biologically active substances into the cytoplasm of immune cells or cancer cells and perform their biological functions precisely and efficiently [15, 16]. Exosomes have numerous advantages as therapeutic drug delivery vehicles, including a small size, good stability, and good biocompatibility and safety. Additionally, they are able to avoid phagocytosis by the reticuloendothelial system (RES) and penetrate deep into a tumor to release drugs by degrading the extracellular matrix [17].
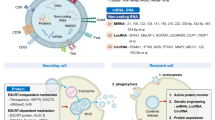
The involvement of exosomes in cancer diagnosis and treatment is one of the current hotspots of cancer research (Fig. 1). An increasing number of studies have found that exosomes play an important role in the occurrence, development, metastasis, detection and treatment of cancer [18,19,20,21,22,23,24]. Comparing cancer cell exosomes to normal cell exosomes revealed that the levels of many of the proteins identified were particularly high in cancer exosomes. This is important because these markers can be used to diagnose exosomes from cancer cells and even identify which tissue they came from. For example, in 2015, a non-small cell lung cancer (NSCLC)-related study, which included blood sample data from 109 patients with stage IIIa-IV NSCLC and 110 controls, found that in the advanced NSCLC patients, CD317 and EGFR were highly expressed on the surface of exosomes [25]. Additionally, exosomes in the blood of treated patients can be monitored to understand the response to cancer treatment. If the exosomes decrease in number or disappear, it may indicate that the treatment is effective. If new mutations are found in exosomes, this could indicate that the cancer is developing new resistance to treatment. Potential tumor detection and treatment markers in exosomes in different tumors are described in detail in Table 2.
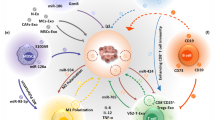
An emerging research area related to exosomes that has gained considerable attention is the application of exosomes in immunotherapy [12]. Exosomes derived from immune cells, tumor cells, and mesenchymal stem cells are the most widely used drug delivery systems [13, 100, 101]. Studies have confirmed that tumor-derived exosomes can carry drugs and target the drugs to tumor cells to inhibit their growth [14, 102,103,104,105,106]. Exosomes released by various immune cells (T cells, DCs, macrophages, etc.) play an important role in immune system regulation [107,108,109,110]. Immune cell-derived exosomes can mimic the characteristics of immune cells targeting tumor cells, conferring therapeutic benefits by attenuating or stimulating immune responses [13, 111]. Therefore, exosomes have great potential in cancer immunotherapy.
Although exosomes have been the subject of many review articles, few reviews have comprehensively summarized the role of exosomes as drug carriers in immunotherapy. In this review, we mainly focus on the application of exosomes for targeted drug delivery in cancer immunotherapy. The characteristics of exosomes as drug carriers and future prospects are also introduced.
2 Exosomes as a drug delivery system
Exosomes are widely found in various body fluids [107, 112]. In vivo, exosomes carry the membrane and cytoplasmic components of the parent cell and play the role of "courier", maintaining the exchange of substances (lipids, proteins, nucleic acids, etc.) between cells [113]. There are various membrane proteins with specific functions on the exosome membrane. For example, CD9 and CD81 help exosomes fuse with recipient cells, CD55 and CD59 protect against complement attack, and CD47 protects against phagocytosis by macrophages [114, 115] (Fig. 2).
Exosomes are naturally nontoxic and highly biocompatible, and they remain in blood circulation for long periods [116, 117]. These unique functions make exosomes a potential ideal drug delivery vehicle. However, autologous- and heterologous-dependent approaches need to be considered when choosing exosomes as drug delivery systems. The study found that the uptake of autologous and heterologous exosomes by recipient cells was significantly different. Autologous exosomes are more biologically similar to their parental cells and may be more suitable for drug delivery [118]. However, heterologous exosomes cannot be completely ignored. The acquisition of heterologous exosomes is often easier than that of autologous exosomes. Studies have found that heterologous exosomes can safely and reliably deliver drugs. Lessi et al. found that primary human macrophage-derived exosomes can be efficiently used for drug delivery [119].
In recent years, exosomes have been found to be a good drug delivery vehicle for cancer treatment [107, 120,121,122]. Several therapeutic approaches based on exosome drug delivery systems have entered clinical trials, as shown in Table 3.
As a drug delivery system, exosomes also face numerous challenges and are affected by many factors. Prof. Gaurav, I. and Thakur, A. systematically reviewed the various factors affecting extracellular vesicle-based drug delivery systems [118]. For example, one issue is exosome isolation and yield. Although techniques for isolating exosomes have been widely reported and commercial extraction kits have been developed, current extraction techniques are still an important limiting factor in the application of exosomes for drug delivery [124]. There is no consensus on a standard procedure for the optimal isolation of exosomes. There are still insufficient technologies to obtain exosomes that can be used in drug delivery systems with high efficiency, high quality and low cost. In addition, exosomal surface modification is an important factor that affects targeted delivery [118]. Chemical modification and genetic engineering are two techniques that can be used for surface modification of exosomes. Surface modifications can affect the delivery capacity and biological effects of exosomes. However, neither is perfect. Due to the complexity of exosome surfaces, chemical modifications often lack site-specific control and even affect the structure and function of the carrier. Genetic engineering is the fusion of the gene sequence of the guide protein or polypeptide with the gene sequence of the selected exosomal membrane protein. This approach is effective for the surface display of polypeptides and proteins but is limited to genetically encoded targeting motifs.
In terms of drug loading, bioactive substances such as proteins, small RNAs and drugs can be loaded into exosomes using chemical methods and genetic engineering techniques [13, 102, 120]. At present, there are two methods to achieve drug loading in exosomes: endogenous loading and exogenous loading.
Endogenous loading, also known as preloading, refers to the loading of drugs into cells before the cells release exosomes. This method turns cells into living factories that release drug-loaded exosomes and directly secrete the desired drug-loaded exosomes. For example, Ran et al. transfected mouse embryonic fibroblasts (NIH3T) with a propeptide-expressing lentivirus (CD63-propeptide-expressing lentivirus), which ultimately enabled the fibroblasts to release exosomes carrying propeptides on their surface [125]. Choi et al. constructed a cell line that stably expressed two recombinant anti-inflammatory proteins, CIBN-EGFP-CD9 and srIκB-mCherry-CRY2. Then, irradiation with blue light (460 nm) induced cells to actively load anti-inflammatory proteins into exosomes [126]. The engineered cells can easily and conveniently produce the target exosomes, which have great potential in the commercialization of exosomal protein therapy. Fu et al. used genetic circuits to reprogram the host liver to direct the synthesis and self-assembly of siRNA into exosomes and facilitate the delivery of siRNA in vivo through circulating exosomes [127].
Exogenous loading, also known as postloading, refers to the processing of exosomes after isolation and purification of natural exosomes. There are various ways to load drugs into exosomes: coincubation, sonication, electroporation, freeze‒thaw, extrusion and permeabilization [16, 121, 128,129,130,131,132,133,134,135,136,137]. Thakur, A et al. successfully loaded two blood‒brain barrier (BBB)-impermeable anticancer drugs, DOX and PTX, into SF7761 stem cell-like GM-derived exosomes with an Exo-Load microfluidic device and found that this treatment exerted a strong tumor growth inhibitory effect [138]. Xu et al. found that exosomes secreted by M1 macrophages provide a proinflammatory environment and that paclitaxel (PTX) encapsulated by coincubation increases the antitumor ability of PTX in breast cancer cells through the caspase-3 pathway [139]. Prof. Alvarez-Erviti used electroporation to load exogenous siRNA into purified exosomes. Intravenous injection of exosomes carrying exogenous siRNA targeted for delivery to oligodendrocytes, microglia, and neurons in the brain results in specific gene knockout [140]. Li et al. used polycarbonate membrane extrusion to fuse drug-encapsulated nanoparticles with exosomes, and the cellular uptake efficiency and antitumor effect of doxorubicin (DOX) were significantly improved [13].
3 Immune cell-derived exosomes
3.1 Dendritic cells
Dendritic cells (DCs) are responsible for processing and presenting antigenic information in vivo [141, 142]. When DCs mature, they have many pseudopods similar to dendrites, so they are called dendritic cells [143]. Mature DCs specialize in processing and presenting various antigenic substances and play a central role in the immune regulation of the human body [144]. They can regulate the body’s humoral immunity, cellular immunity and tumor immunity. Through the processing of tumor cells, DCs can activate human T lymphocytes and enhance the phagocytosis of T lymphocytes on tumor cells, thereby exerting an effective antitumor effect [145]. DC-derived exosomes (Dexs) are able to enhance immune responses by transferring MHC complexes from antigen-exposed to unexposed DCs [111, 146]. These DCs load processed antigen onto major histocompatibility complex I and II (MHCI and MHCII) molecules, present to naïve CD8+ and CD4+ T cells, respectively, and transmit antigen memory to T cells [100]. Mature DCs are able to carry more intercellular adhesion molecule-1 (ICAM-1) and MHCII and exert stronger T-cell stimulation [147, 148].
In a phase II clinical trial, investigators used second-generation Dex (IFN-γ-Dex) for maintenance immunotherapy in patients with advanced non-small cell lung cancer (NSCLC). The study included 22 patients, and the primary endpoint was progression-free survival (PFS) 4 months after chemotherapy was stopped. This phase II clinical trial demonstrated the ability of Dex to enhance the antitumor immune response of NK cells in patients with advanced NSCLC [109]. Zhen et al. reported that alpha-fetoprotein (AFP)-expressing Dex could induce potent antigen-specific immune responses in ectopic or orthotopic hepatocellular carcinoma (HCC) mice, improved the immune microenvironment of autologous tumors, and decrease the amount of immune stimulation cell and CD8+ CTL infiltration, levels of immunosuppressive cytokines and the number of Treg cells [149]. A recent study reported that Dex vaccine (DEXP&A2&N) promoted the recruitment and activation of DCs in mice with liver cancer, thereby enhancing tumor-specific immune responses [150]. Studies have reported that DC cell-derived exosomes can cross the BBB to deliver RNAi to the brain and play a biological role, such as inhibiting tumor growth [151]. Xu et al. reported that fluorouracil could be encapsulated in DC cell-derived exosomes by electroporation and found that FU-DC-Exos had a strong inhibitory effect on the proliferation of colon cancer cells [152]. Using the property of rabies virus glycoprotein (RVG) to specifically bind to nicotinic acetylcholine receptors (AchR) on neurons and BBB vascular endothelium, Lakhal et al. established membrane-expressing LAMP2B-RVG exosomes (derived from DCs) for targeted delivery [151]. In conclusion, DC-derived exosomes offer great promise for cancer therapy as drug delivery vehicles.
3.2 Macrophages
Macrophages are specialized, long-lived phagocytic cells of the innate immune system [153, 154]. They are the largest immune cell population in solid tumors and play an important role in maintaining homeostasis [155]. Macrophages have two main polarization states: the proinflammatory M1 phenotype and the anti-inflammatory and reparative M2 phenotype [156]. Macrophages can regulate their microenvironment and provide instructions to neighboring cells to maintain balance. Studies have found that macrophages can not only inhibit tumor growth and progression but also promote tumor cell growth, survival, and angiogenesis through an immunosuppressive microenvironment [156,157,158]. Feng et al. developed a macrophage-derived exosome-coated poly(lactic-glycolic acid) nanoplatform for targeted chemotherapy in triple-negative breast cancer (TNBC). This engineered exosome was found to have a significant tumor-targeting effect and to improve the cellular uptake efficiency and antitumor efficacy of doxorubicin [13]. M1 macrophage-derived exosomes (M1-exos) have been demonstrated to deliver anticancer drugs for cancer therapy. Kim et al. found that exosome membrane reorganization under the action of ultrasound could improve drug loading efficiency and sustained drug release. Therefore, the ultrasonic method was used to load PTX into M1-exos, and the results confirmed that M1-exos-PTX has a significant therapeutic effect on lung cancer [137]. Cianciaruso et al. found that macrophage-secreted exosomes have molecular features related to Th1/M1 polarization and enhance inflammatory and immune responses [159]. TAM-EVs also contain bioactive lipids and biosynthetic enzymes that may alter proinflammatory signaling in cancer cells. Therefore, although studies have found that macrophages can promote the malignant progression of tumors by stimulating angiogenesis, increasing tumor cell invasion and metastasis, and inhibiting antitumor immunity, the exosomes they secrete may stimulate rather than limit antitumor immunity [158, 159].
In conclusion, the potential of macrophage-derived exosomes for cell-to-cell communication in oncology research is unclear. Since macrophages are the largest immune cell population in solid tumors, the status and importance of macrophage-derived exosomes in future cancer research cannot be underestimated.
3.3 T-lymphocytes
T lymphocytes are important immune cells in the body that fight diseases such as infections and tumors [160,161,162]. T lymphocytes include three types: natural killer T cells (NKT), T helper (Th) lymphocytes, and regulatory T cells (Tregs) [163]. Their functions include: (1) killing and eliminating virus-infected cells and cancer cells via cytotoxicity; (2) secreting cytokines to regulate the role of other immune cells; and (3) distinguishing exogenous pathogenic antigens and self-antigens to prevent inappropriate autoimmune responses. Qiu et al. found that PD-1 carried by T-cell-derived exosomes could interact with PD-L1 on the distal cell surface or exosomes. The internalization of PD-L1 is induced by endocytosis, preventing the binding of PD-L1 to PD-1 and thereby inhibiting the occurrence of immune escape [162].
Chimeric antigen receptors-modified T cells (CAR-T) have emerged as a promising new type of immunotherapy [164,165,166,167]. Johnson et al. used CAR-T cells to deliver RN7SL1, an endogenous RNA, to activate RIG-I/MDA5 signaling, stimulate a characteristic dendritic cell (DC) subset, and improve immune function [168]. Studies have found that CAR-T cells can release CAR-carrying exosomes and that CAR-expressing exosomes can significantly inhibit tumor growth, which may become a new antitumor therapy in the future [169]. Yang et al. found that exosomes derived from mesothelin (MSLN)-targeted CAR-T cells maintained the characteristics of parental T cells, such as CD3 expression on the membrane surface [170]. CAR-carrying exosomes can significantly inhibit the malignant progression of TNBC [170]. In conclusion, T-cell-derived exosomes are important mediators involved in immune regulation, and their application as drug delivery vehicles in cancer therapy is still in the exploratory stage.
3.4 Natural killer cells (NK cells)
Natural killer cells (NK) are important immune cells that are related to antitumor and immune regulation [171]. NK cells can exert cytotoxic effects on a variety of cells, destroying infectious and tumor cells in the absence of antigen presentation [172]. NK-cell-derived exosomes (NK-Exos) contain the same molecules that kill cancer cells; they are much smaller than NK cells and are better able to penetrate tumors [173]. Exosomes secreted by NK cells also have a tumor-homing ability [110].
Kang et al. found that samples from patients with NSCLC contained more NK cells and NK-Exos, which were correlated with the number of circulating tumor cells (CTCs) [173]. CD56 and FLOT1 expressed by NK-Exos can be recognized and taken up by cancer cells, leading to cytotoxic death of cancer cells [173]. NK-Exos are also cytotoxic to melanoma cells and induce melanoma cell apoptosis. FasL inhibitors attenuate NK-Exos cytotoxic effects on melanoma. In vivo experiments in mice also showed that tumor size was significantly reduced after NK-Exos treatment [113]. NK-Exos have cytotoxic effects on tumors and good application prospects in cancer immunotherapy. miR-3607-3p in NK-Exos can target IL-26, thereby inhibiting the proliferation, invasion and migration of pancreatic cancer cells [174]. Taken together, these results show that NK-cell-derived exosomes are very promising in the field of tumor therapy, and the substances with antitumor effects that they carry are expected to become a promising new cancer treatment.
4 Tumor cells-derived exosomes
Tumor-derived exosomes (TEXs) promote tumor growth and development in many ways, affect the differentiation and activation of immune cells and regulate antigen presentation [104, 175,176,177,178]. TEXs are key mediators of intercellular communication, can remodel distant microenvironments, such as premetastatic niches, and play an important role in the distant metastasis of tumors [106, 179, 180]. David et al. found that the tumor exosomal CEMIP protein can act on cerebral blood vessels and microglia, remodel the brain microenvironment, and promote the metastasis of cancer cells to the brain [181]. Tumor-derived exosomal miR-1247-3p directly targets B4GALT3, induces activation of the β1-integrin-NF-κB signaling pathway in cancer-associated fibroblasts, and promotes lung metastasis of liver cancer [104]. Studies have found that glioblastoma cell (GM)-derived exosomes can spread to systemic biological fluids through the BBB, which is considered to be an effective biomarker for the discovery of tracking glioma progression [8, 182]. GM-derived exosomes can penetrate the BBB, making it possible to deliver drugs that cannot penetrate the BBB to intracranial tumors. In addition, studies have reported that hypoxia increases the expression of MCT1 and CD147 in GMs, which leads to changes in the biological characteristics of exosomes released by GMs and affects the uptake of exosomes by receptor cells (e.g., endothelial cells) [182].
The diverse biological characteristics of TEXs make them effective molecular markers and therapeutic targets, especially for immunotherapy. Tumor cell-derived exosomal miR-21 and miR-29a can bind to TLR8 and TLR7 in immune cells, leading to the activation of NF-κB and the secretion of inflammatory factors [101]. NSCLC cell-derived exosomal circUSP7 suppresses CD8+ T-cell function by upregulating SHP2 expression by sponging miR-934, thereby promoting the resistance of NSCLC patients to anti-PD1 immunotherapy [105]. Samantha et al. found that primary tumor-derived exosomes can induce tissue-resident macrophages in the premetastatic microenvironment to upregulate the immunosuppressive molecule PD-L1 and secrete high levels of lactate, thereby establishing an immunosuppressive microenvironment that promotes tumor metastasis [176]. These research results confirm the regulatory effect of tumor-derived exosomes on the immune system and provide new targets for tumor immunotherapy.
Mauro et al. found that tumor-derived exosomes carrying PDL1 to lymph nodes can inhibit the function of T cells. Knocking out the TRAMP-C2 gene inhibited the release of exosomes from tumor cells, which in turn inhibited tumor growth. These results are in contrast to those obtained by the injection of exosomes carrying PDL1 collected in vitro [183]. Some studies have also found that tumor-derived exosome-loaded drugs can reduce the number of cancer cells [14, 102, 103]. This study confirms the important value of targeted inhibition of tumor-derived exosomes in tumor immunotherapy. In-depth exploration of the immunoregulatory mechanism of tumor-derived exosomes on various immune cells will help guide immunotherapy and overcome the resistance of current immune checkpoint inhibitors.
5 Mesenchymal stem cells-derived exosomes
Mesenchymal stem cells (MSCs) are a type of pluripotent stem cell that have all the commonalities of stem cells, namely, self-renewal and multidirectional differentiation capabilities. MSCs exist not only in bone marrow but also in skeletal muscle, periosteum, and trabecular bone [184]. Mesenchymal stem cell-secreted exosomes (MSC-Exos) possess not only the tumor-regulating properties of parental cells but also the ability to transport valuable cargoes (such as proteins, lipids, RNAs) across physiological barriers to target cells and play a role in communication and regulation [185]. A recent study found that MSC-Exos can affect the occurrence and development of tumor cells by promotion or inhibition in two ways [186]. Wang et al. found that MSC-Exos could transfer miRNA-221 to HGC27 gastric cancer cells, thereby promoting the growth and migration of tumor cells [187]. Zhang et al. found that bone marrow mesenchymal stem cell-derived exosomes (BMSC-exos) carry miR-193a-3p, miR-210-3p and miR-5100 to recipient cells, activate the STAT3 signaling pathway to induce epithelial-mesenchymal transition, and enhance the invasive ability of lung cancer cells [188]. In addition, a study found that exosomal miR-145 derived from adipose MSCs inhibited prostate cancer growth by reducing Bcl-xl activity and promoting tumor cell apoptosis [189].
MSC-Exos have a certain targeting ability, and the modification of MSC-Exos can target the tumor site and have a stronger anticancer effect. Kamerkar et al. found that in a mouse model of pancreatic cancer, loading specific siRNA or shRNA carrying the oncogene KRAS into MSC-Exos significantly enhanced its efficacy and improved overall survival depending on CD47 [123]. Bagheri et al. used MSC-Exos as carriers to transport RNA, protein and small-molecule drugs to specific parts of the tumor for tumor therapy. For example, doxorubicin loaded into MSC-Exos by electroporation inhibited colon cancer growth. This mode of administration can significantly increase the accumulation of doxorubicin in tumor tissue [184, 190].
MSC-Exos still have considerable research potential and broad application prospects in the field of in vivo drug delivery, which can provide new research methods and ideas for cancer treatment. However, the research conclusions are still in the preclinical stage, and more in-depth basic research is needed to clarify its molecular mechanism in the future.
6 Conclusions and prospects
In this review, we discuss the application value of exosome-based drug delivery systems, as well as the recent progress and application prospects of exosomes derived from immune cells, tumor cells and mesenchymal stem cells in the field of cancer immunotherapy. Exosomes can be released into the extracellular environment by immune cells or cancer cells. An increasing number of studies have shown that exosomes have important regulatory effects on the immune system [13, 107, 111]. Some biologically active substances on exosomes, such as MHC and costimulatory molecules, have been shown to be involved in exosome-mediated anticancer immune responses. A more comprehensive and in-depth understanding of the molecular mechanism of exosomes in immune regulation is of considerable importance for the development of anticancer immunotherapy based on exosome drug delivery systems.
Multiple studies have confirmed engineered exosomes to be an important tool for drug delivery, and multiple clinical trials are underway. For example, Thakur, A's team successfully loaded BBB-impermeable anticancer drugs into SF7761 stem cell-like GM-derived exosomes with an Exo-Load microfluidic device and inhibited tumor growth [138]. However, to facilitate the true application of exosomes in the clinic, there are still some hurdles that need to be further addressed. For example, exosome surface modification directly affects the efficiency of drug delivery. Although chemical modification and genetic engineering, two techniques that can be used for surface modification of exosomes, are widely used, both still have shortcomings. In the future, advances in exosome surface modification technology are crucial for the application of exosome-based drug delivery systems. In addition. isolating high-purity living NK-cell populations and extracting exosomes from these cells also face technical difficulties. At present, the extraction method of exosomes is mainly ultracentrifugation; however, the extraction yield is low, the cost is high, and it is difficult to achieve industrial production and large-scale clinical application [191]. Large-scale production and storage, biodistribution and heterogeneity, and engineered processing are all prominent challenges that must be overcome for clinical applications. At the same time, maintaining the stability and functionality of exosomes, the targeted therapeutic effects and the side effects of exosomes are also issues that must be considered.
In conclusion, we have made significant progress in understanding the biological properties of exosomes and their applications in the field of cancer over the past decade. Exosome-mediated drug delivery is expected to overcome important challenges in therapeutic areas, for example, drug delivery across biological barriers such as the BBB, and the use of patient tissue-derived exosomes as personalized and biocompatible therapeutic drug delivery vectors. However, there is still a long way to go before we can fully understand all the molecular mechanisms associated with exosomes and apply them in the clinic.
Data availability
Not applicable.
Abbreviations
- MHC:
-
Major histocompatibility complex
- ILVs:
-
Intraluminal vesicles
- MVEs:
-
Multivesicular endosomes
- ESCRT:
-
Endosomal sorting complex
- RES:
-
Reticuloendothelial system
- PTX:
-
Paclitaxel
- DCs:
-
Dendritic cells
- Dex:
-
DCs-derived exosomes
- ICAM-1:
-
Intercellular adhesion molecule-1
- NSCLC:
-
Non-small cell lung cancer
- PFS:
-
Progression-free survival
- AFP:
-
Alpha-fetoprotein
- HCC:
-
Hepatocellular carcinoma
- TNBC:
-
Triple-negative breast cancer
- M1-exos:
-
M1 macrophage-derived exosomes
- NKT:
-
Natural killer T cells
- Th:
-
T helper
- T regs:
-
Regulatory T cells
- CARs:
-
Chimeric antigen receptors
- MSLN:
-
Mesothelin
- NK:
-
Natural killer cells
- NK-Exos:
-
NK cell-derived exosomes
- CTCs:
-
Circulating tumor cells
- TEXs:
-
Tumor-derived exosomes
- MSCs:
-
Mesenchymal stem cells
- MSCs-Exo:
-
Mesenchymal stem cells-secreted exosomes
- BMSCs-exo:
-
Bone marrow mesenchymal stem cell-derived exosomes
References
Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645(1):63–70. https://doi.org/10.1016/0005-2736(81)90512-5.
Johnstone RM, Adam M, Hammond JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020. https://doi.org/10.1126/science.aau6977.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. https://doi.org/10.1146/annurev-biochem-013118-111902.
Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21(1):77–91. https://doi.org/10.1016/j.devcel.2011.05.015.
Pfitzner AK, Mercier V, Jiang X, et al. An ESCRT-III polymerization sequence drives membrane deformation and fission. Cell. 2020;182(5):1140-1155 e1118. https://doi.org/10.1016/j.cell.2020.07.021.
Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. https://doi.org/10.1038/ncb2000 (sup pp 11-13).
Thakur A, Ke X, Chen YW, et al. The mini player with diverse functions: extracellular vesicles in cell biology, disease, and therapeutics. Protein Cell. 2022;13(9):631–54. https://doi.org/10.1007/s13238-021-00863-6.
Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744–62. https://doi.org/10.1016/j.cmet.2021.08.006.
Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. https://doi.org/10.1038/s41392-020-00261-0.
Menck K, Scharf C, Bleckmann A, et al. Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J Mol Cell Biol. 2015;7(2):143–53. https://doi.org/10.1093/jmcb/mju047.
Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19(1):160. https://doi.org/10.1186/s12943-020-01278-3.
Li S, Wu Y, Ding F, et al. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale. 2020;12(19):10854–62. https://doi.org/10.1039/d0nr00523a.
Gutierrez-Millan C, Calvo Diaz C, Lanao JM, Colino CI. Advances in exosomes-based drug delivery systems. Macromol Biosci. 2021;21(1): e2000269. https://doi.org/10.1002/mabi.202000269.
Park O, Choi ES, Yu G, et al. Efficient delivery of tyrosinase related protein-2 (TRP2) peptides to lymph nodes using serum-derived exosomes. Macromol Biosci. 2018;18(12): e1800301. https://doi.org/10.1002/mabi.201800301.
Yong T, Zhang X, Bie N, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10(1):3838. https://doi.org/10.1038/s41467-019-11718-4.
Yong T, Wang D, Li X, et al. Extracellular vesicles for tumor targeting delivery based on five features principle. J Control Release. 2020;322:555–65. https://doi.org/10.1016/j.jconrel.2020.03.039.
Becker A, Thakur BK, Weiss JM, et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–48. https://doi.org/10.1016/j.ccell.2016.10.009.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24. https://doi.org/10.1038/nrm3838.
Wu H, Fu M, Liu J, et al. The role and application of small extracellular vesicles in gastric cancer. Mol Cancer. 2021;20(1):71. https://doi.org/10.1186/s12943-021-01365-z.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49(3):347–60. https://doi.org/10.1016/j.devcel.2019.04.011.
Steinbichler TB, Dudas J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–81. https://doi.org/10.1016/j.semcancer.2017.02.006.
Casari I, Howard JA, Robless EE, Falasca M. Exosomal integrins and their influence on pancreatic cancer progression and metastasis. Cancer Lett. 2021;507:124–34. https://doi.org/10.1016/j.canlet.2021.03.010.
Shi J, Zhang Y, Yao B, et al. Role of exosomes in the progression, diagnosis, and treatment of gliomas. Med Sci Monit. 2020;26: e924023. https://doi.org/10.12659/msm.924023.
Jakobsen KR, Paulsen BS, Baek R, et al. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles. 2015;4:26659. https://doi.org/10.3402/jev.v4.26659.
Wang B, Mao JH, Wang BY, et al. Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-kappaB signaling pathway. Cancer Lett. 2020;489:87–99. https://doi.org/10.1016/j.canlet.2020.05.038.
Guo L, Zhu Y, Li L, et al. Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer Med. 2019;8(12):5687–701. https://doi.org/10.1002/cam4.2454.
Wang T, Ning K, Lu TX, et al. Increasing circulating exosomes-carrying TRPC5 predicts chemoresistance in metastatic breast cancer patients. Cancer Sci. 2017;108(3):448–54. https://doi.org/10.1111/cas.13150.
Lee SJ, Lee J, Jung JH, et al. Exosomal Del-1 as a potent diagnostic marker for breast cancer: prospective cohort study. Clin Breast Cancer. 2021;21(6):e748–56. https://doi.org/10.1016/j.clbc.2021.02.002.
Gu P, Sun M, Li L, et al. Breast tumor-derived exosomal microRNA-200b-3p promotes specific organ metastasis through regulating CCL2 expression in lung epithelial cells. Front Cell Dev Biol. 2021;9: 657158. https://doi.org/10.3389/fcell.2021.657158.
Zang X, Gu J, Zhang J, et al. Exosome-transmitted lncRNA UFC1 promotes non-small-cell lung cancer progression by EZH2-mediated epigenetic silencing of PTEN expression. Cell Death Dis. 2020;11(4):215. https://doi.org/10.1038/s41419-020-2409-0.
Wan L, Chen X, Deng J, et al. Plasma exosome-derived B-cell translation gene 1: a predictive marker for the prognosis in patients with non-small cell lung cancer. J Cancer. 2021;12(5):1538–47. https://doi.org/10.7150/jca.52320.
Sun S, Chen H, Xu C, et al. Exosomal miR-106b serves as a novel marker for lung cancer and promotes cancer metastasis via targeting PTEN. Life Sci. 2020;244: 117297. https://doi.org/10.1016/j.lfs.2020.117297.
Kang Y, You J, Gan Y, et al. Serum and serum exosomal CircRNAs hsa_circ_0001492, hsa_circ_0001439, and hsa_circ_0000896 as diagnostic biomarkers for lung adenocarcinoma. Front Oncol. 2022;12: 912246. https://doi.org/10.3389/fonc.2022.912246.
Ding C, Xi G, Wang G, et al. Exosomal Circ-MEMO1 promotes the progression and aerobic glycolysis of non-small cell lung cancer through targeting MiR-101-3p/KRAS axis. Front Genet. 2020;11:962. https://doi.org/10.3389/fgene.2020.00962.
Chen L, Huang S, Huang J, et al. Role and mechanism of exosome-derived long noncoding RNA HOTAIR in lung cancer. ACS Omega. 2021;6(27):17217–27. https://doi.org/10.1021/acsomega.1c00905.
Piao HY, Guo S, Wang Y, Zhang J. Exosome-transmitted lncRNA PCGEM1 promotes invasive and metastasis in gastric cancer by maintaining the stability of SNAI1. Clin Transl Oncol. 2021;23(2):246–56. https://doi.org/10.1007/s12094-020-02412-9.
McAtee CO, Booth C, Elowsky C, et al. Prostate tumor cell exosomes containing hyaluronidase Hyal1 stimulate prostate stromal cell motility by engagement of FAK-mediated integrin signaling. Matrix Biol. 2019;78–79:165–79. https://doi.org/10.1016/j.matbio.2018.05.002.
Logozzi M, Angelini DF, Iessi E, et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017;403:318–29. https://doi.org/10.1016/j.canlet.2017.06.036.
Li Q, Hu J, Shi Y, et al. Exosomal lncAY927529 enhances prostate cancer cell proliferation and invasion through regulating bone microenvironment. Cell Cycle. 2021;20(23):2531–46. https://doi.org/10.1080/15384101.2021.1992853.
Kawakami K, Fujita Y, Matsuda Y, et al. Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer. 2017;17(1):316. https://doi.org/10.1186/s12885-017-3301-x.
Horie K, Kawakami K, Fujita Y, et al. Serum exosomal gamma-glutamyltransferase activity increased in patients with renal cell carcinoma with advanced clinicopathological features. Oncology. 2020;98(10):734–42. https://doi.org/10.1159/000508688.
Duijvesz D, Versluis CY, van der Fels CA, et al. Immuno-based detection of extracellular vesicles in urine as diagnostic marker for prostate cancer. Int J Cancer. 2015;137(12):2869–78. https://doi.org/10.1002/ijc.29664.
Barcelo M, Castells M, Bassas L, et al. Semen miRNAs contained in exosomes as non-invasive biomarkers for prostate cancer diagnosis. Sci Rep. 2019;9(1):13772. https://doi.org/10.1038/s41598-019-50172-6.
Jiang Y, Ji X, Liu K, et al. Exosomal miR-200c-3p negatively regulates the migraion and invasion of lipopolysaccharide (LPS)-stimulated colorectal cancer (CRC). BMC Mol Cell Biol. 2020;21(1):48. https://doi.org/10.1186/s12860-020-00291-0.
Okuda Y, Shimura T, Iwasaki H, et al. Serum exosomal dicer is a useful biomarker for early detection of differentiated gastric adenocarcinoma. Digestion. 2021;102(4):640–9. https://doi.org/10.1159/000510993.
Pan S, Deng Y, Fu J, et al. N6methyladenosine upregulates miR181d5p in exosomes derived from cancerassociated fibroblasts to inhibit 5FU sensitivity by targeting NCALD in colorectal cancer. Int J Oncol. 2022. https://doi.org/10.3892/ijo.2022.5304.
Yan S, Jiang Y, Liang C, et al. Exosomal miR-6803-5p as potential diagnostic and prognostic marker in colorectal cancer. J Cell Biochem. 2018;119(5):4113–9. https://doi.org/10.1002/jcb.26609.
Liu T, Zhang X, Du L, et al. Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol Cancer. 2019;18(1):43. https://doi.org/10.1186/s12943-019-0981-7.
Shang A, Gu C, Wang W, et al. Exosomal circPACRGL promotes progression of colorectal cancer via the miR-142-3p/miR-506-3p- TGF-beta1 axis. Mol Cancer. 2020;19(1):117. https://doi.org/10.1186/s12943-020-01235-0.
Zhao K, Cheng X, Ye Z, et al. Exosome-mediated transfer of circ_0000338 enhances 5-fluorouracil resistance in colorectal cancer through regulating microRNA 217 (miR-217) and miR-485-3p. Mol Cell Biol. 2021. https://doi.org/10.1128/MCB.00517-20.
Ning T, Li J, He Y, et al. Exosomal miR-208b related with oxaliplatin resistance promotes Treg expansion in colorectal cancer. Mol Ther. 2021;29(9):2723–36. https://doi.org/10.1016/j.ymthe.2021.04.028.
Cho WC, Kim M, Park JW, et al. Exosomal miR-193a and let-7g accelerate cancer progression on primary colorectal cancer and paired peritoneal metastatic cancer. Transl Oncol. 2021;14(2): 101000. https://doi.org/10.1016/j.tranon.2020.101000.
Jiang L, Zhang Y, Guo L, et al. Exosomal microRNA-107 reverses chemotherapeutic drug resistance of gastric cancer cells through HMGA2/mTOR/P-gp pathway. BMC Cancer. 2021;21(1):1290. https://doi.org/10.1186/s12885-021-09020-y.
Li S, Zhang M, Zhang H, et al. Exosomal long noncoding RNA lnc-GNAQ-6:1 may serve as a diagnostic marker for gastric cancer. Clin Chim Acta. 2020;501:252–7. https://doi.org/10.1016/j.cca.2019.10.047.
Ye W, Deng X, Fan Y. Exosomal miRNA423-5p mediated oncogene activity in papillary thyroid carcinoma: a potential diagnostic and biological target for cancer therapy. Neoplasma. 2019;66(4):516–23. https://doi.org/10.4149/neo_2018_180824N643.
Cai C, Zhang H, Zhu Y, et al. Serum exosomal long noncoding RNA pcsk2-2:1 as a potential novel diagnostic biomarker for gastric cancer. Onco Targets Ther. 2019;12:10035–41. https://doi.org/10.2147/OTT.S229033.
Miki Y, Yashiro M, Okuno T, et al. Clinico-pathological significance of exosome marker CD63 expression on cancer cells and stromal cells in gastric cancer. PLoS ONE. 2018;13(9): e0202956. https://doi.org/10.1371/journal.pone.0202956.
Carli ALE, Afshar-Sterle S, Rai A, et al. Cancer stem cell marker DCLK1 reprograms small extracellular vesicles toward migratory phenotype in gastric cancer cells. Proteomics. 2021;21(13–14): e2000098. https://doi.org/10.1002/pmic.202000098.
Guo S, Hu C, Zhai X, Sun D. Circular RNA 0006602 in plasma exosomes: a new potential diagnostic biomarker for hepatocellular carcinoma. Am J Transl Res. 2021;13(6):6001–15.
Li Z, Yanfang W, Li J, et al. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237–50. https://doi.org/10.1016/j.canlet.2018.04.035.
Tian F, Wang P, Lin D, et al. Exosome-delivered miR-221/222 exacerbates tumor liver metastasis by targeting SPINT1 in colorectal cancer. Cancer Sci. 2021;112(9):3744–55. https://doi.org/10.1111/cas.15028.
Yokota Y, Noda T, Okumura Y, et al. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci. 2021;112(3):1275–88. https://doi.org/10.1111/cas.14807.
Xu H, Dong X, Chen Y, Wang X. Serum exosomal hnRNPH1 mRNA as a novel marker for hepatocellular carcinoma. Clin Chem Lab Med. 2018;56(3):479–84. https://doi.org/10.1515/cclm-2017-0327.
Liu X, Cheng D, Cui M, et al. Exosome marker proteins of tumor-associated fibroblasts and exosome-derived miR-92a-3p act as potential biomarkers for liver cancer. Crit Rev Eukaryot Gene Expr. 2022;32(1):49–57. https://doi.org/10.1615/CritRevEukaryotGeneExpr.2021039570.
Fang JH, Zhang ZJ, Shang LR, et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68(4):1459–75. https://doi.org/10.1002/hep.29920.
Cui Y, Xu HF, Liu MY, et al. Mechanism of exosomal microRNA-224 in development of hepatocellular carcinoma and its diagnostic and prognostic value. World J Gastroenterol. 2019;25(15):1890–8. https://doi.org/10.3748/wjg.v25.i15.1890.
Mao Y, Zhang L, Li Y. Application of CircEIF4G2 in screening of cervical lesions. Clin Lab. 2020. https://doi.org/10.7754/Clin.Lab.2019.190915.
You X, Wang Y, Meng J, et al. Exosomal miR663b exposed to TGFbeta1 promotes cervical cancer metastasis and epithelialmesenchymal transition by targeting MGAT3. Oncol Rep. 2021. https://doi.org/10.3892/or.2021.7963.
Zhang Y, Cai H, Chen S, et al. Exosomal transfer of miR-124 inhibits normal fibroblasts to cancer-associated fibroblasts transition by targeting sphingosine kinase 1 in ovarian cancer. J Cell Biochem. 2019;120(8):13187–201. https://doi.org/10.1002/jcb.28593.
Zhang X, Sheng Y, Li B, et al. Ovarian cancer derived PKR1 positive exosomes promote angiogenesis by promoting migration and tube formation in vitro. Cell Biochem Funct. 2021;39(2):308–16. https://doi.org/10.1002/cbf.3583.
Xiong J, He X, Xu Y, et al. MiR-200b is upregulated in plasma-derived exosomes and functions as an oncogene by promoting macrophage M2 polarization in ovarian cancer. J Ovarian Res. 2021;14(1):74. https://doi.org/10.1186/s13048-021-00826-9.
Cao J, Zhang Y, Mu J, et al. Exosomal miR-21-5p contributes to ovarian cancer progression by regulating CDK6. Hum Cell. 2021;34(4):1185–96. https://doi.org/10.1007/s13577-021-00522-2.
Liu T, Li P, Li J, et al. Exosomal and intracellular miR-320b promotes lymphatic metastasis in esophageal squamous cell carcinoma. Mol Ther Oncolytics. 2021;23:163–80. https://doi.org/10.1016/j.omto.2021.09.003.
Huang TY, Wang CY, Chen KY, Huang LT. Urinary exosomal thyroglobulin in thyroid cancer patients with post-ablative therapy: a new biomarker in thyroid cancer. Front Endocrinol. 2020;11:382. https://doi.org/10.3389/fendo.2020.00382.
Wen J, Yang T, Mallouk N, et al. Urinary exosomal CA9 mRNA as a novel liquid biopsy for molecular diagnosis of bladder cancer. Int J Nanomedicine. 2021;16:4805–11. https://doi.org/10.2147/IJN.S312322.
Wang J, Yang K, Yuan W, Gao Z. Determination of serum exosomal H19 as a noninvasive biomarker for bladder cancer diagnosis and prognosis. Med Sci Monit. 2018;24:9307–16. https://doi.org/10.12659/MSM.912018.
Xu L, Faruqu FN, Lim YM, et al. Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials. 2021;264: 120369. https://doi.org/10.1016/j.biomaterials.2020.120369.
Wu Y, Zeng H, Yu Q, et al. A circulating exosome RNA signature is a potential diagnostic marker for pancreatic cancer, a systematic study. Cancers. 2021. https://doi.org/10.3390/cancers13112565.
Wang L, Wu J, Ye N, et al. Plasma-derived exosome MiR-19b acts as a diagnostic marker for pancreatic cancer. Front Oncol. 2021;11: 739111. https://doi.org/10.3389/fonc.2021.739111.
Sun H, Rana S, Wang Z, et al. The pancreatic cancer-initiating cell marker CD44v6 affects transcription, translation, and signaling: consequences for exosome composition and delivery. J Oncol. 2019;2019:3516973. https://doi.org/10.1155/2019/3516973.
Lux A, Kahlert C, Grutzmann R, Pilarsky C. c-Met and PD-L1 on circulating exosomes as diagnostic and prognostic markers for pancreatic cancer. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20133305.
Liu P, Kong L, Jin H, et al. Differential secretome of pancreatic cancer cells in serum-containing conditioned medium reveals CCT8 as a new biomarker of pancreatic cancer invasion and metastasis. Cancer Cell Int. 2019;19:262. https://doi.org/10.1186/s12935-019-0980-1.
Li X, Li K, Li M, et al. Chemoresistance transmission via exosome-transferred MMP14 in pancreatic cancer. Front Oncol. 2022;12: 844648. https://doi.org/10.3389/fonc.2022.844648.
Kyuno D, Zhao K, Schnolzer M, et al. Claudin7-dependent exosome-promoted reprogramming of nonmetastasizing tumor cells. Int J Cancer. 2019;145(8):2182–200. https://doi.org/10.1002/ijc.32312.
Harada T, Yamamoto H, Kishida S, et al. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017;108(1):42–52. https://doi.org/10.1111/cas.13109.
Goto T, Fujiya M, Konishi H, et al. An elevated expression of serum exosomal microRNA-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer. 2018;18(1):116. https://doi.org/10.1186/s12885-018-4006-5.
Chen K, Wang Q, Liu X, et al. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. Int J Biol Sci. 2022;18(3):1220–37. https://doi.org/10.7150/ijbs.67675.
Fang Z, Wang X, Wu J, et al. High serum extracellular vesicle miR-10b expression predicts poor prognosis in patients with acute myeloid leukemia. Cancer Biomark. 2020;27(1):1–9. https://doi.org/10.3233/CBM-190211.
Hrdinova T, Toman O, Dresler J, et al. Exosomes released by imatinibresistant K562 cells contain specific membrane markers, IFITM3, CD146 and CD36 and increase the survival of imatinibsensitive cells in the presence of imatinib. Int J Oncol. 2021;58(2):238–50. https://doi.org/10.3892/ijo.2020.5163.
Liu Y, Fu W, Cao X, et al. Delivery of miR-224-5p by exosomes from cancer-associated fibroblasts potentiates progression of clear cell renal cell carcinoma. Comput Math Methods Med. 2021;2021:5517747. https://doi.org/10.1155/2021/5517747.
Li G, Mallouk N, Flandrin P, et al. Presence of urinary exosomes for liquid biopsy of clear cell renal cell carcinoma: protocol for a pilot feasibility study. JMIR Res Protoc. 2021;10(7): e24423. https://doi.org/10.2196/24423.
Choi DY, Park JN, Paek SH, et al. Detecting early-stage malignant melanoma using a calcium switch-enriched exosome subpopulation containing tumor markers as a sample. Biosens Bioelectron. 2022;198: 113828. https://doi.org/10.1016/j.bios.2021.113828.
Guo Y, Zhang X, Wang L, et al. The plasma exosomal miR-1180-3p serves as a novel potential diagnostic marker for cutaneous melanoma. Cancer Cell Int. 2021;21(1):487. https://doi.org/10.1186/s12935-021-02164-8.
Felicetti F, De Feo A, Coscia C, et al. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J Transl Med. 2016;14:56. https://doi.org/10.1186/s12967-016-0811-2.
Cordonnier M, Nardin C, Chanteloup G, et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9(1):1710899. https://doi.org/10.1080/20013078.2019.1710899.
Wang X, Cao Q, Shi Y, et al. Identification of low-dose radiation-induced exosomal circ-METRN and miR-4709-3p/GRB14/PDGFRalpha pathway as a key regulatory mechanism in Glioblastoma progression and radioresistance: Functional validation and clinical theranostic significance. Int J Biol Sci. 2021;17(4):1061–78. https://doi.org/10.7150/ijbs.57168.
Wang J, Li T, Wang B. Exosomal transfer of miR253p promotes the proliferation and temozolomide resistance of glioblastoma cells by targeting FBXW7. Int J Oncol. 2021. https://doi.org/10.3892/ijo.2021.5244.
Vaidya M, Bacchus M, Sugaya K. Differential sequences of exosomal NANOG DNA as a potential diagnostic cancer marker. PLoS ONE. 2018;13(5): e0197782. https://doi.org/10.1371/journal.pone.0197782.
Thery C, Amigorena S. The cell biology of antigen presentation in dendritic cells. Curr Opin Immunol. 2001;13(1):45–51. https://doi.org/10.1016/s0952-7915(00)00180-1.
Fabbri M, Paone A, Calore F, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109(31):E2110-2116. https://doi.org/10.1073/pnas.1209414109.
Walker S, Busatto S, Pham A, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9(26):8001–17. https://doi.org/10.7150/thno.37097.
Li Y, Gao Y, Gong C, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine. 2018;14(7):1973–85. https://doi.org/10.1016/j.nano.2018.05.020.
Fang T, Lv H, Lv G, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9(1):191. https://doi.org/10.1038/s41467-017-02583-0.
Chen SW, Zhu SQ, Pei X, et al. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol Cancer. 2021;20(1):144. https://doi.org/10.1186/s12943-021-01448-x.
Zhao S, Mi Y, Guan B, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13(1):156. https://doi.org/10.1186/s13045-020-00991-2.
Thakur A, Parra DC, Motallebnejad P, et al. Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater. 2022;10:281–94. https://doi.org/10.1016/j.bioactmat.2021.08.029.
Yan W, Jiang S. Immune cell-derived exosomes in the cancer-immunity cycle. Trends Cancer. 2020;6(6):506–17. https://doi.org/10.1016/j.trecan.2020.02.013.
Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5(4): e1071008. https://doi.org/10.1080/2162402X.2015.1071008.
Wu F, Xie M, Hun M, et al. Natural killer cell-derived extracellular vesicles: novel players in cancer immunotherapy. Front Immunol. 2021;12: 658698. https://doi.org/10.3389/fimmu.2021.658698.
Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–93. https://doi.org/10.1038/nri2567.
Zhu Q, Cheng L, Deng C, et al. The genetic source tracking of human urinary exosomes. Proc Natl Acad Sci USA. 2021. https://doi.org/10.1073/pnas.2108876118.
Zhu L, Kalimuthu S, Gangadaran P, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7(10):2732–45. https://doi.org/10.7150/thno.18752.
Clayton A, Harris CL, Court J, et al. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003;33(2):522–31. https://doi.org/10.1002/immu.200310028.
Ohnami N, Nakamura A, Miyado M, et al. CD81 and CD9 work independently as extracellular components upon fusion of sperm and oocyte. Biol Open. 2012;1(7):640–7. https://doi.org/10.1242/bio.20121420.
Luan X, Sansanaphongpricha K, Myers I, et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–63. https://doi.org/10.1038/aps.2017.12.
Kahroba H, Hejazi MS, Samadi N. Exosomes: from carcinogenesis and metastasis to diagnosis and treatment of gastric cancer. Cell Mol Life Sci. 2019;76(9):1747–58. https://doi.org/10.1007/s00018-019-03035-2.
Gaurav I, Thakur A, Iyaswamy A, et al. Factors affecting extracellular vesicles based drug delivery systems. Molecules. 2021. https://doi.org/10.3390/molecules26061544.
Iessi E, Logozzi M, Lugini L, et al. Acridine orange/exosomes increase the delivery and the effectiveness of acridine orange in human melanoma cells: a new prototype for theranostics of tumors. J Enzyme Inhib Med Chem. 2017;32(1):648–57. https://doi.org/10.1080/14756366.2017.1292263.
Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–95. https://doi.org/10.7150/thno.52570.
Khani AT, Sharifzad F, Mardpour S, et al. Tumor extracellular vesicles loaded with exogenous Let-7i and miR-142 can modulate both immune response and tumor microenvironment to initiate a powerful anti-tumor response. Cancer Lett. 2021;501:200–9. https://doi.org/10.1016/j.canlet.2020.11.014.
Elliott RO, He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics. 2021. https://doi.org/10.3390/pharmaceutics13010122.
Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. https://doi.org/10.1038/nature22341.
Sato YT, Umezaki K, Sawada S, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933. https://doi.org/10.1038/srep21933.
Ran N, Gao X, Dong X, et al. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials. 2020;236: 119826. https://doi.org/10.1016/j.biomaterials.2020.119826.
Choi H, Kim Y, Mirzaaghasi A, et al. Exosome-based delivery of super-repressor IkappaBalpha relieves sepsis-associated organ damage and mortality. Sci Adv. 2020;6(15):eaaz6980. https://doi.org/10.1126/sciadv.aaz6980.
Fu Z, Zhang X, Zhou X, et al. In vivo self-assembled small RNAs as a new generation of RNAi therapeutics. Cell Res. 2021;31(6):631–48. https://doi.org/10.1038/s41422-021-00491-z.
Chakravarti AR, Pacelli S, Paul A. Investigation of human adipose stem cell-derived nanoparticles as a biomimetic carrier for intracellular drug delivery. Nanoscale. 2020;12(47):24273–84. https://doi.org/10.1039/d0nr06571d.
Li S, Stockl S, Lukas C, et al. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1beta-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res Ther. 2021;12(1):252. https://doi.org/10.1186/s13287-021-02317-6.
Kim MS, Haney MJ, Zhao Y, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14(1):195–204. https://doi.org/10.1016/j.nano.2017.09.011.
Xie F, Zhou X, Fang M, et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci. 2019;6(24):1901779. https://doi.org/10.1002/advs.201901779.
Koh E, Lee EJ, Nam GH, et al. Exosome-SIRPalpha, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–9. https://doi.org/10.1016/j.biomaterials.2017.01.004.
Wahlgren J, De LKT, Brisslert M, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17): e130. https://doi.org/10.1093/nar/gks463.
Zhou W, Zhou Y, Chen X, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268: 120546. https://doi.org/10.1016/j.biomaterials.2020.120546.
Liang Y, Xu X, Li X, et al. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–47. https://doi.org/10.1021/acsami.0c10458.
Rountree RB, Mandl SJ, Nachtwey JM, et al. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Cancer Res. 2011;71(15):5235–44. https://doi.org/10.1158/0008-5472.Can-10-4076.
Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–64. https://doi.org/10.1016/j.nano.2015.10.012.
Thakur A, Sidu RK, Zou H, et al. Inhibition of glioma cells’ proliferation by doxorubicin-loaded exosomes via microfluidics. Int J Nanomedicine. 2020;15:8331–43. https://doi.org/10.2147/IJN.S263956.
Xu Z, Zhang C, Yu Y, et al. Photoactive silver nanoagents for backgroundless monitoring and precision killing of multidrug-resistant bacteria. Nanotheranostics. 2021;5(4):472–87. https://doi.org/10.7150/ntno.62364.
Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5. https://doi.org/10.1038/nbt.1807.
Wculek SK, Cueto FJ, Mujal AM, et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. https://doi.org/10.1038/s41577-019-0210-z.
Gardner A, de Mingo PA, Ruffell B. Dendritic cells and their role in immunotherapy. Front Immunol. 2020;11:924. https://doi.org/10.3389/fimmu.2020.00924.
Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–77. https://doi.org/10.1038/nrc3258.
Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6(6):476–83. https://doi.org/10.1038/nri1845.
Le Gall CM, Weiden J, Eggermont LJ, Figdor CG. Dendritic cells in cancer immunotherapy. Nat Mater. 2018;17(6):474–5. https://doi.org/10.1038/s41563-018-0093-6.
Gao D, Jiang L. Exosomes in cancer therapy: a novel experimental strategy. Am J Cancer Res. 2018;8(11):2165–75.
Colino J, Snapper CM. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J Immunol. 2006;177(6):3757–62. https://doi.org/10.4049/jimmunol.177.6.3757.
Segura E, Nicco C, Lombard B, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106(1):216–23. https://doi.org/10.1182/blood-2005-01-0220.
Lu Z, Zuo B, Jing R, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67(4):739–48. https://doi.org/10.1016/j.jhep.2017.05.019.
Zuo B, Zhang Y, Zhao K, et al. Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses. J Hematol Oncol. 2022;15(1):46. https://doi.org/10.1186/s13045-022-01266-8.
Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays. 2011;33(10):737–41. https://doi.org/10.1002/bies.201100076.
Xu M, Chen Q, Li J, et al. Dendritic cell-derived exosome-entrapped fluorouracil can enhance its anti-colon cancer effect. J BUON. 2020;25(3):1413–22.
Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–75. https://doi.org/10.1146/annurev-immunol-032414-112220.
Sheu KM, Hoffmann A. Functional hallmarks of healthy macrophage responses: their regulatory basis and disease relevance. Annu Rev Immunol. 2022;40:295–321. https://doi.org/10.1146/annurev-immunol-101320-031555.
Tian L, Lei A, Tan T, et al. Macrophage-based combination therapies as a new strategy for cancer immunotherapy. Kidney Dis. 2022;8(1):26–43. https://doi.org/10.1159/000518664.
DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–82. https://doi.org/10.1038/s41577-019-0127-6.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. https://doi.org/10.1016/j.cell.2010.03.014.
Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904. https://doi.org/10.1038/nrd.2018.169.
Cianciaruso C, Beltraminelli T, Duval F, et al. Molecular profiling and functional analysis of macrophage-derived tumor extracellular vesicles. Cell Rep. 2019;27(10):3062-3080 e3011. https://doi.org/10.1016/j.celrep.2019.05.008.
Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–81. https://doi.org/10.1038/nri3191.
Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545(7655):423–31. https://doi.org/10.1038/nature22395.
Qiu Y, Yang Y, Yang R, et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene. 2021;40(31):4992–5001. https://doi.org/10.1038/s41388-021-01896-1.
Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell. 2018;33(4):547–62. https://doi.org/10.1016/j.ccell.2018.03.012.
Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38(4):473–88. https://doi.org/10.1016/j.ccell.2020.07.005.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. https://doi.org/10.1038/s41408-021-00459-7.
Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21(3):e168–78. https://doi.org/10.1016/S1470-2045(19)30823-X.
Liu D. CAR-T “the living drugs”, immune checkpoint inhibitors, and precision medicine: a new era of cancer therapy. J Hematol Oncol. 2019;12(1):113. https://doi.org/10.1186/s13045-019-0819-1.
Johnson LR, Lee DY, Eacret JS, et al. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell. 2021;184(19):4981-4995 e4914. https://doi.org/10.1016/j.cell.2021.08.004.
Fu W, Lei C, Liu S, et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 2019;10(1):4355. https://doi.org/10.1038/s41467-019-12321-3.
Yang P, Cao X, Cai H, et al. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cell Immunol. 2021;360: 104262. https://doi.org/10.1016/j.cellimm.2020.104262.
Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. SnapShot: natural killer cells. Cell. 2020;180(6):1280-1280 e1281. https://doi.org/10.1016/j.cell.2020.02.029.
Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. 2016;16(5):310–20. https://doi.org/10.1038/nri.2016.34.
Kang YT, Niu Z, Hadlock T, et al. On-chip biogenesis of circulating NK cell-derived exosomes in non-small cell lung cancer exhibits antitumoral activity. Adv Sci. 2021;8(6):2003747. https://doi.org/10.1002/advs.202003747.
Sun H, Shi K, Qi K, et al. Natural killer cell-derived exosomal miR-3607-3p inhibits pancreatic cancer progression by targeting IL-26. Front Immunol. 2019;10:2819. https://doi.org/10.3389/fimmu.2019.02819.
Czernek L, Duchler M. Functions of cancer-derived extracellular vesicles in immunosuppression. Arch Immunol Ther Exp. 2017;65(4):311–23. https://doi.org/10.1007/s00005-016-0453-3.
Morrissey SM, Zhang F, Ding C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021;33(10):2040-2058 e2010. https://doi.org/10.1016/j.cmet.2021.09.002.
Olejarz W, Dominiak A, Zolnierzak A, et al. Tumor-derived exosomes in immunosuppression and immunotherapy. J Immunol Res. 2020;2020:6272498. https://doi.org/10.1155/2020/6272498.
Wang B, Tan Z, Guan F. Tumor-derived exosomes mediate the instability of cadherins and promote tumor progression. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20153652.
Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. https://doi.org/10.1038/nature15756.
Wang X, Luo G, Zhang K, et al. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res. 2018;78(16):4586–98. https://doi.org/10.1158/0008-5472.CAN-17-3841.
Rodrigues G, Hoshino A, Kenific CM, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. 2019;21(11):1403–12. https://doi.org/10.1038/s41556-019-0404-4.
Thakur A, Qiu G, Xu C, et al. Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma. Sci Adv. 2020;6(26):eaaz6119. https://doi.org/10.1126/sciadv.aaz6119.
Poggio M, Hu T, Pai CC, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414-427 e413. https://doi.org/10.1016/j.cell.2019.02.016.
Zhang F, Guo J, Zhang Z, et al. Mesenchymal stem cell-derived exosome: a tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2022;526:29–40. https://doi.org/10.1016/j.canlet.2021.11.015.
Lai RC, Tan SS, Yeo RW, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. https://doi.org/10.3402/jev.v5.29828.
Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. https://doi.org/10.1038/s41556-018-0250-9.
Wang M, Zhao C, Shi H, et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110(5):1199–210. https://doi.org/10.1038/bjc.2014.14.
Zhang X, Sai B, Wang F, et al. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18(1):40. https://doi.org/10.1186/s12943-019-0959-5.
Takahara K, Ii M, Inamoto T, et al. microRNA-145 mediates the inhibitory effect of adipose tissue-derived stromal cells on prostate cancer. Stem Cells Dev. 2016;25(17):1290–8. https://doi.org/10.1089/scd.2016.0093.
Bagheri E, Abnous K, Farzad SA, et al. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020;261: 118369. https://doi.org/10.1016/j.lfs.2020.118369.
Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. https://doi.org/10.3402/jev.v4.27031.
Acknowledgements
Not applicable.
Funding
This work was supported by the Beijing Municipal Science & Technology Commission (No. Z171100001017077).
Author information
Authors and Affiliations
Contributions
ZF, YD and ZX: writing, figures and tables; PL and JL: writing, review and supervision; FL: supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, Z., Ding, Y., Xue, Z. et al. Roles of exosomes as drug delivery systems in cancer immunotherapy: a mini-review. Discov Onc 13, 74 (2022). https://doi.org/10.1007/s12672-022-00539-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-022-00539-5