Abstract
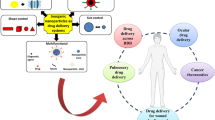
Nanoparticles have promisingly served as therapeutic agents in drug delivery systems to supress side effects while improving the effectiveness of treatment to deadly cancers. The present study aims to fabricate chitosan/agarose/graphene oxide/montmorillonite nanocomposites to serve as drug carriers for the prolonged release of curcumin to suppress MCF7 breast cancer cells. Offering a range of advantages, including biocompatibility and pH-sensitivity, the nanocomposites were synthesized via a water-in-oil-in-water (W/O/W) emulsification technique. Characterization studies were conducted with FTIR and XRD analyses. Dynamic light scattering (DLS) analysis was conducted to measure the mean size of the produced nanoscale emulsions, with zeta potential assessments further performed to characterize the nanocomposites in terms of surface charge. FE-SEM imagery indicated the uniform and flat surface of the synthesized nanocomposites. MTT assay results showed that the nanocomposites were most toxic to MCF7 breast cancer cells. The occurrence of apoptosis to MCF7 breast cancer cells was evident from the flow cytometry findings. Implementing a dialysis procedure, the curcumin was found to be released at a higher rate in acidic media. Altogether, our findings proved that the designed CS/AG/GO/MMT@CUR nanocomposites could be a drug carrier for treating breast cancer cells.
Graphical Abstract










Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
Notes
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
References
Aghebati-Maleki, A., Dolati, S., Ahmadi, M., Baghbanzhadeh, A., Asadi, M., Fotouhi, A., Yousefi, M., & Aghebati-Maleki, L. (2020). Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. Journal of Cellular Physiology, 235(3), 1962–1972. https://doi.org/10.1002/jcp.29126
Hanahan, D. (2022). Hallmarks of cancer: New dimensions. Cancer Discovery, 12(1), 31–46. https://doi.org/10.1158/2159-8290.CD-21-1059
Sheikh, A., Shadab, Md., & Kesharwani, P. (2022). Aptamer grafted nanoparticle as targeted therapeutic tool for the treatment of breast cancer. Biomedicine & Pharmacotherapy, 146, 112530. https://doi.org/10.1016/j.biopha.2021.112530
Ma, X., & Herbert, Yu. (2006). Cancer issue: Global burden of cancer. The Yale Journal of Biology and Medicine, 79(3–4), 85.
Aboud, K., Meissner, M., Ocen, J., & Jones, R. (2023). Cytotoxic chemotherapy: Clinical aspects. Medicine. https://doi.org/10.1016/j.mpmed.2022.10.005
Petrelli, N. J., Winer, E. P., Brahmer, J., Dubey, S., Smith, S., Thomas, C., Vahdat, L. T., Obel, J., Vogelzang, N., Markman, M., Sweetenham, J. W., Pfister, D., Kris, M. G., Schuchter, L. M., Sawaya, R., Raghavan, D., Ganz, P. A., & Kramer, B. (2009). Clinical cancer advances 2009: Major research advances in cancer treatment, prevention, and screening–A report from the American Society of Clinical Oncology. Journal of Clinical Oncology, 27, 6052–6069.
Dong, P., Rakesh, K. P., Manukumar, H. M., Mohammed, Y. H. E., Karthik, C. S., Sumathi, S., Mallu, P., & Qin, H.-L. (2019). Innovative nano-carriers in anticancer drug delivery-A comprehensive review. Bioorganic Chemistry, 85, 325–336. https://doi.org/10.1016/j.bioorg.2019.01.019
Zadeh, E. S., Ghanbari, N., Salehi, Z., Derakhti, S., Amoabediny, G., Akbari, M., & Tokmedash, M. A. (2023). Smart pH-responsive magnetic graphene quantum dots nanocarriers for anticancer drug delivery of curcumin. Materials Chemistry and Physics, 127336. https://doi.org/10.1016/j.matchemphys.2023.127336
Wicki, A., Witzigmann, D., Balasubramanian, V., & Huwyler, J. (2015). Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. Journal of Controlled Release, 200, 138–157. https://doi.org/10.1016/j.jconrel.2014.12.030
Shi, J., Kantoff, P. W., Wooster, R., & Farokhzad, O. C. (2017). Cancer nanomedicine: Progress, challenges and opportunities. Nature Reviews Cancer, 17(1), 20–37.
Senapati, S., Mahanta, A. K., Kumar, S., & Maiti, P. (2018). Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduction and Targeted Therapy, 3(1), 7.
Vinothini, K., Rajendran, N. K., Ramu, A., Elumalai, N., & Rajan, M. (2019). Folate receptor targeted delivery of paclitaxel to breast cancer cells via folic acid conjugated graphene oxide grafted methyl acrylate nanocarrier. Biomedicine & Pharmacotherapy, 110, 906–917. https://doi.org/10.1016/j.biopha.2018.12.008
Pooresmaeil, M., & Namazi, H. (2020). pH-sensitive ternary Fe3O4/GQDs@ G hybrid microspheres; Synthesis, characterization and drug delivery application. Journal of Alloys and Compounds, 846, 156419. https://doi.org/10.1016/j.jallcom.2020.156419
Zhao, J., Zhang, Z., & Wang, X. (2020). Fabrication of pH-responsive PAA-NaMnF3@ DOX hybrid nanostructures for magnetic resonance imaging and drug delivery. Journal of Alloys and Compounds, 820, 153142. https://doi.org/10.1016/j.jallcom.2019.153142
Xie, L., Ji, X., Zhang, Qi., & Wei, Y. (2022). Curcumin combined with photodynamic therapy, promising therapies for the treatment of cancer. Biomedicine & Pharmacotherapy, 146, 112567. https://doi.org/10.1016/j.biopha.2021.112567
Kazemi, S., Pourmadadi, M., Yazdian, F., & Ghadami, A. (2021). The synthesis and characterization of targeted delivery curcumin using chitosan-magnetite-reduced graphene oxide as nano-carrier. International Journal of Biological Macromolecules, 186, 554–562. https://doi.org/10.1016/j.ijbiomac.2021.06.184
Samadi, A., Haseli, S., Pourmadadi, M., Rashedi, H., Yazdian, F., & Navaei-Nigjeh, M. (2020). Curcumin-loaded chitosan-agarose-montmorillonite hydrogel nanocomposite for the treatment of breast cancer. In 2020 27th National and 5th International Iranian Conference on Biomedical Engineering (ICBME) (pp. 148–153). IEEE. https://doi.org/10.1109/ICBME51989.2020.9319425
Kumar, S., Kesharwani, S. S., Mathur, H., Tyagi, M., Bhat, G. J., & Tummala, H. (2016). Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin. European Journal of Pharmaceutical Sciences, 82, 86–96. https://doi.org/10.1016/j.ejps.2015.11.010
Esthar, S., Rajesh, J., Prakash, N., Ayyanaar, S., Bhaskar, R., Thanigaivel, S., Webster, T., & Rajagopal, G. (2023). An effective biodegradable curcumin loaded magnetic microsphere: Applications for drug delivery and cancer treatment. Pharmacological Research-Modern Chinese Medicine, 100219. https://doi.org/10.1016/j.prmcm.2023.100219
Javanbakht, S., Pooresmaeil, M., & Namazi, H. (2019). Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohydrate polymers, 208, 294–301. https://doi.org/10.1016/j.carbpol.2018.12.066
Sedghi, R., Ashrafzadeh, S., & Heidari, B. (2021). pH-sensitive molecularly imprinted polymer based on graphene oxide for stimuli actuated controlled release of curcumin. Journal of Alloys and Compounds, 857, 157603. https://doi.org/10.1016/j.jallcom.2020.157603
Nazir, S., Khan, M. U. A., Al-Arjan, W. S., AbdRazak, S. I., Javed, A., & Kadir, M. R. A. (2021). Nanocomposite hydrogels for melanoma skin cancer care and treatment: In-vitro drug delivery, drug release kinetics and anti-cancer activities. Arabian Journal of Chemistry, 14(5), 103120. https://doi.org/10.1016/j.arabjc.2021.103120
de Oliveira, É. C., et al. (2022). In vitro and in vivo safety profile assessment of graphene oxide decorated with different concentrations of magnetite. Journal of Nanoparticle Research, 24(7), 150. https://doi.org/10.1007/s11051-022-05529-w
Karimi, M., Eslami, M., Sahandi-Zangabad, P., Mirab, F., Farajisafiloo, N., Shafaei, Z., Ghosh, D., Bozorgomid, M., Dashkhaneh, F., & Hamblin, M. R. (2016). pH-sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 8(5), 696–716. https://doi.org/10.1002/wnan.1389
Wang, P.-J., Zhou, Di., Guo, H.-H., Liu, W.-F., Jin-Zhan, Su., Mao-Sen, Fu., Singh, C., Trukhanov, S., & Trukhanov, A. (2020). Ultrahigh enhancement rate of the energy density of flexible polymer nanocomposites using core–shell BaTiO 3@ MgO structures as the filler. Journal of Materials Chemistry A, 8(22), 11124–11132. https://doi.org/10.1039/d0ta03304a
da Rosa Salles, T., et al. (2022). Magnetic nanocrystalline cellulose: Azithromycin adsorption and in vitro biological activity against melanoma cells. Journal of Polymers and the Environment, 30(7), 2695–2713. https://doi.org/10.1007/s10924-022-02388-3
Yakovenko, O. S., Yu Matzui, L., Vovchenko, L. L., Oliynyk, V. V., Trukhanov, A. V., Trukhanov, S. V., Borovoy, M. O., et al. (2020). Effect of magnetic fillers and their orientation on the electrodynamic properties of BaFe 12–x Ga x O 19 (x= 0.1–1.2)—Epoxy composites with carbon nanotubes within GHz range. Applied Nanoscience, 10, 4747–4752. https://doi.org/10.1007/s13204-020-01477-w
Sun, X., Liu, C., Omer, A. M., Yang, L.-Y., & Ouyang, X.-K. (2019). Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. International Journal of Biological Macromolecules, 132, 487–494. https://doi.org/10.1016/j.ijbiomac.2019.03.225
Bruckmann, F. D. S., et al. (2022). Influence of magnetite incorporation into chitosan on the adsorption of the methotrexate and in vitro cytotoxicity. Environmental Science and Pollution Research, 29(46), 70413–70434. https://doi.org/10.1007/s11356-022-20786-x
Dubey, S. K., Bhatt, T., Agrawal, M., Saha, R. N., Saraf, S., Saraf, S., & Alexander, A. (2022). Application of chitosan modified nanocarriers in breast cancer. International Journal of Biological Macromolecules, 194, 521–538. https://doi.org/10.1016/j.ijbiomac.2021.11.095
Pompeu, L. D., et al. (2023). Evaluation of cytotoxicity, reactive oxygen species and nitrous oxide of nanochitosan from shrimp shell. International Journal of Biological Macromolecules, 235, 123730. https://doi.org/10.1016/j.ijbiomac.2023.123730
Dong, Y., Li, S., Li, X., & Wang, X. (2021). Smart MXene/agarose hydrogel with photothermal property for controlled drug release. International Journal of Biological Macromolecules, 190, 693–699. https://doi.org/10.1016/j.ijbiomac.2021.09.037
Qi, X., Su, T., Tong, X., Xiong, W., Zeng, Q., Qian, Y., Zhou, Z., Wu, X., Li, Z., Shen, L., He, X., Xu, C., Chen, M., Li, Y., & Shen, J. (2019). Facile formation of salecan/agarose hydrogels with tunable structural properties for cell culture. Carbohydrate Polymers, 224, 11. https://doi.org/10.1016/j.carbpol.2019.115208
Ninan, N., Forget, A., Shastri, V. P., Voelcker, N. H., & Blencowe, A. (2016). Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Applied Materials & Interfaces, 8(42), 28511–28521. https://doi.org/10.1021/acsami.6b10491
Yazdi, M. K., Taghizadeh, A., Taghizadeh, M., Stadler, F. J., Farokhi, M., Mottaghitalab, F., Zarrintaj, P., et al. (2020). Agarose-based biomaterials for advanced drug delivery. Journal of Controlled Release, 326, 523–543. https://doi.org/10.1016/j.jconrel.2020.07.028
Park, J.-H., Shin, H.-J., Kim, M. H., Kim, J.-S., Kang, N., Lee, J.-Y., Kim, K.-T., Lee, J. I., & Kim, D.-D. (2016). Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. Journal of Pharmaceutical Investigation, 46, 363–375. https://doi.org/10.1007/s40005-016-0258-8
Gârea, S. A., Voicu, A. I., & Iovu, H. (2017). Clay–polymer nanocomposites for controlled drug release. In Clay-polymer nanocomposites (pp. 475–509). Elsevier. https://doi.org/10.1016/B978-0-323-46153-5.00014-8
Olad, A., Hagh, H. B. K., Mirmohseni, A., & Azhar, F. F. (2019). Graphene oxide and montmorillonite enriched natural polymeric scaffold for bone tissue engineering. Ceramics International, 45(12), 15609–15619. https://doi.org/10.1016/j.ceramint.2019.05.071
Bozoğlan, B. K., Duman, O., & Tunç, S. (2020). Preparation and characterization of thermosensitive chitosan/carboxymethylcellulose/scleroglucan nanocomposite hydrogels. International Journal of Biological Macromolecules, 162, 781–797. https://doi.org/10.1016/j.ijbiomac.2020.06.087
Rutakhli, A., Sabahi, H., & Riazi, G. H. (2019). Nanocomposite of montmorillonite/nettle extract: A potential ingredient for functional foods development. Journal of Functional Foods, 57, 166–172. https://doi.org/10.1016/j.jff.2019.04.004
Sabzevari, A. G., Sabahi, H., & Nikbakht, M. (2020). Montmorillonite, a natural biocompatible nanosheet with intrinsic antitumor activity. Colloids and Surfaces B: Biointerfaces, 190, 110884. https://doi.org/10.1016/j.colsurfb.2020.110884
Zhu, Y., Kong, G., Pan, Y., Liu, L., Yang, Bo., Zhang, S., Lai, D., & Che, C. (2022). An improved Hummers method to synthesize graphene oxide using much less concentrated sulfuric acid. Chinese Chemical Letters. https://doi.org/10.1016/j.cclet.2022.01.060
Kumar, S. R., Priyatharshni, S., Babu, V. N., Mangalaraj, D., Viswanathan, C., Kannan, S., & Ponpandian, N. (2014). Quercetin conjugated superparamagnetic magnetite nanoparticles for in-vitro analysis of breast cancer cell lines for chemotherapy applications. Journal of Colloid and Interface Science, 436, 234–242. https://doi.org/10.1016/j.jcis.2014.08.064
Jahanizadeh, S., Yazdian, F., Marjani, A., Omidi, M., & Rashedi, H. (2017). Curcumin-loaded chitosan/carboxymethyl starch/montmorillonite bio-nanocomposite for reduction of dental bacterial biofilm formation. International Journal of Biological Macromolecules, 105, 757–763. https://doi.org/10.1016/j.ijbiomac.2017.07.101
Rezagholizade-shirvan, A., Najafi, M. F., Behmadi, H., & Masrournia, M. (2022). Preparation of nano-composites based on curcumin/chitosan-PVA-alginate to improve stability, antioxidant, antibacterial and anticancer activity of curcumin. Inorganic Chemistry Communications, 145, 110022. https://doi.org/10.1016/j.inoche.2022.110022
Melo-Silveira, R. F., Fidelis, G. P., Costa, M. S. S. P., Telles, C. B. S., Dantas-Santos, N., de Oliveira, Elias S., Ribeiro, V. B., et al. (2011). In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs. International Journal of Molecular Sciences, 13(1), 409–426. https://doi.org/10.3390/ijms13010409
Gerami, S. E., Pourmadadi, M., Fatoorehchi, H., Yazdian, F., Rashedi, H., & Nigjeh, M. N. (2021). Preparation of pH-sensitive chitosan/polyvinylpyrrolidone/α-Fe2O3 nanocomposite for drug delivery application: Emphasis on ameliorating restrictions. International Journal of Biological Macromolecules, 173, 409–420. https://doi.org/10.1016/j.ijbiomac.2021.01.067
Wolkers, W. F., Oliver, A. E., Tablin, F., & Crowe, J. H. (2004). A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydrate Research, 339(6), 1077–1085. https://doi.org/10.1016/j.carres.2004.01.016
Silva, F. R. F., Dore, C. M. P. G., Marques, C. T., Nascimento, M. S., Benevides, N. M. B., Rocha, H. A. O., Chavante, S. F., & Leite, E. L. (2010). Anticoagulant activity, paw edema and pleurisy induced carrageenan: Action of major types of commercial carrageenans. Carbohydrate Polymers, 79(1), 26–33. https://doi.org/10.1016/j.carbpol.2009.07.010
Queiroz, M. F., Melo, K. R. T., Sabry, D. A., Sassaki, G. L., & Rocha, H. A. O. (2014). Does the use of chitosan contribute to oxalate kidney stone formation? Marine Drugs, 13(1), 141–158. https://doi.org/10.3390/md13010141
Han, D., Yan, L., Chen, W., & Li, W. (2011). Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydrate Polymers, 83(2), 653–658. https://doi.org/10.1016/j.carbpol.2010.08.038
Zarei, Z., & Akhlaghinia, B. (2018). Cu(II) immobilized on Fe3O4@Agarose nanomagnetic catalyst functionalized with ethanolamine phosphate–salicylaldehyde schiff base: A magnetically reusable nanocatalyst for preparation of 2-substituted imidazolines, oxazolines, and thiazolines. Turkish Journal of Chemistry, 42, 170–191. https://doi.org/10.3906/kim-1704-53
Song, J., Wang, X., & Chang, C-T. (2014). Preparation and characterization of graphene oxide. Journal of Nanomaterials, 2014. https://doi.org/10.1155/2014/276143
Sun, X., Liu, Z., Welsher, K., Robinson, J. T., Goodwin, A., Zaric, S., & Dai, H. (2008). Nano-graphene oxide for cellular imaging and drug delivery. Nano Research, 1, 203–212. https://doi.org/10.1007/s12274-008-8021-8
Li, X., Feng, J., Yaping, Du., Bai, J., Fan, H., Zhang, H., Peng, Y., & Li, F. (2015). One-pot synthesis of CoFe 2 O 4/graphene oxide hybrids and their conversion into FeCo/graphene hybrids for lightweight and highly efficient microwave absorber. Journal of Materials Chemistry A, 3(10), 5535–5546. https://doi.org/10.1039/c0xx00000x
Sivashankari, P. R., & Prabaharan, M. (2020). Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. International Journal of Biological Macromolecules, 146, 222–231. https://doi.org/10.1016/j.ijbiomac.2019.12.219
Shehap, A. M., Nasr, R. A., Mahfouz, M. A., & Ismail, A. M. (2021). Preparation and characterizations of high doping chitosan/MMT nanocomposites films for removing iron from ground water. Journal of Environmental Chemical Engineering, 9(1), 104700. https://doi.org/10.1016/j.jece.2020.104700
Reddy, O. S., Subha, M. C. S., Jithendra, T., Madhavi, C., & Rao, K. C. (2021). Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. Journal of Pharmaceutical Analysis, 11(2), 191–199. https://doi.org/10.1016/j.jpha.2020.07.002
Khodaei, A., Jahanmard, F., Hosseini, H. R. M., Bagheri, R., Dabbagh, A., Weinans, H., & Yavari, S. A. (2022). Controlled temperature-mediated curcumin release from magneto-thermal nanocarriers to kill bone tumors. Bioactive Materials, 11, 107–117. https://doi.org/10.1016/j.bioactmat.2021.09.028
Pooresmaeil, M., Asl, E. A., & Namazi, H. (2021). A new pH-sensitive CS/Zn-MOF@ GO ternary hybrid compound as a biofriendly and implantable platform for prolonged 5- fluorouracil delivery to human breast cancer cells. Journal of Alloys and Compounds, 885, 160992. https://doi.org/10.1016/j.jallcom.2021.160992
Shi, D., Karmakar, B., Osman, H.-E.H., El-kott, A. F., Morsy, K., & Abdel-Daim, M. M. (2022). Design and synthesis of chitosan/agar/Ag NPs: A potent and green bio-nanocomposite for the treatment of glucocorticoid induced osteoporosis in rats. Arabian Journal of Chemistry, 15(1), 103471. https://doi.org/10.1016/j.arabjc.2021.103471
Sujiono, E. H., Zabrian, D., Dahlan, M. Y., Amin, B. D., & Agus, J. (2020). Graphene oxide based coconut shell waste: Synthesis by modified Hummers method and characterization. Heliyon, 6(8), e04568. https://doi.org/10.1016/j.heliyon.2020.e04568
Prabha, G., & Raj, V. (2016). Preparation and characterization of chitosan - Polyethylene glycol-polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 104, 808–816. https://doi.org/10.1002/jbm.b.33637
Doost, A. S., Kassozi, V., Grootaert, C., Claeys, M., Dewettinck, K., Van Camp, J., & Van der Meeren, P. (2019). Self-assembly, functionality, and in-vitro properties of quercetin loaded nanoparticles based on shellac-almond gum biological macromolecules. International Journal of Biological Macromolecules, 129, 1024–1033. https://doi.org/10.1016/j.ijbiomac.2019.02.071
Joseph, E., & Singhvi, G. (2019). Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. Nanomaterials for Drug Delivery and Therapy, 91–116. https://doi.org/10.1016/B978-0-12-816505-8.00007-2
Wang, H., Gong, X., Guo, X., Liu, C., Fan, Y.-Y., Zhang, J., Niu, B., & Li, W. (2019). Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. International Journal of Biological Macromolecules, 121, 1118–1125. https://doi.org/10.1016/j.ijbiomac.2018.10.121
Sampath, M., Pichaimani, A., Kumpati, P., & Sengottuvelan, B. (2020). The remarkable role of emulsifier and chitosan, dextran and PEG as capping agents in the enhanced delivery of curcumin by nanoparticles in breast cancer cells. International Journal of Biological Macromolecules, 162, 748–761. https://doi.org/10.1016/j.ijbiomac.2020.06.188
Elbialy, N. S., Aboushoushah, S. F., & Mohamed, N. (2022). Bioinspired synthesis of protein/polysaccharide-decorated folate as a nanocarrier of curcumin to potentiate cancer therapy. International Journal of Pharmaceutics, 613, 121420. https://doi.org/10.1016/j.ijpharm.2021.121420
Abdel-Hakeem, M. A., Mongy, S., Hassan, B., Tantawi, O. I., & Badawy, I. (2021). Curcumin loaded chitosan-protamine nanoparticles revealed antitumor activity via suppression of NF-κB, proinflammatory cytokines and Bcl-2 gene expression in the breast cancer cells. Journal of Pharmaceutical Sciences, 110(9), 3298–3305. https://doi.org/10.1016/j.xphs.2021.06.004
HeydariForoushani, P., Rahmani, E., Alemzadeh, I., Vossoughi, M., Pourmadadi, M., Rahdar, A., & Díez-Pascual, A. M. (2022). Curcumin sustained release with a hybrid chitosan-silk fibroin nanofiber containing silver nanoparticles as a novel highly efficient antibacterial wound dressing. Nanomaterials, 12(19), 3426. https://doi.org/10.3390/nano12193426
Pourmadadi, M., Ahmadi, M., & Yazdian, F. (2023). Synthesis of a novel pH-responsive Fe3O4/chitosan/agarose double nanoemulsion as a promising nanocarrier with sustained release of curcumin to treat MCF-7 cell line. International Journal of Biological Macromolecules, 235, 123786. https://doi.org/10.1016/j.ijbiomac.2023.123786
Razi, M. A., Wakabayashi, R., Tahara, Y., Goto, M., & Kamiya, N. (2018). Genipin-stabilized caseinate-chitosan nanoparticles for enhanced stability and anti-cancer activity of curcumin”. Colloids and Surfaces B: Biointerfaces, 164, 308–315. https://doi.org/10.1016/j.colsurfb.2018.01.041
Karami, M. H., Pourmadadi, M., Abdouss, M., Kalaee, M. R., Moradi, O., Rahdar, A., & Díez-Pascual, A. M. (2023). Novel chitosan/γ-alumina/carbon quantum dot hydrogel nanocarrier for targeted drug delivery. International Journal of Biological Macromolecules, 251, 126280. https://doi.org/10.1016/j.ijbiomac.2023.126280
Jafari, H., & Namazi, H. (2023). pH-sensitive biosystem based on laponite RD/chitosan/polyvinyl alcohol hydrogels for controlled delivery of curcumin to breast cancer cells. Colloids and Surfaces B: Biointerfaces, 231, 113585. https://doi.org/10.1016/j.colsurfb.2023.113585
Haseli, S., Pourmadadi, M., Samadi, A., Yazdian, F., Abdouss, M., Rashedi, H., & Navaei-Nigjeh, M. (2022). A novel pH-responsive nanoniosomal emulsion for sustained release of curcumin from a chitosan-based nanocarrier: Emphasis on the concurrent improvement of loading, sustained release, and apoptosis induction. Biotechnology Progress, 38(5), e3280. https://doi.org/10.1002/btpr.3280
Funding
This work was supported by the Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Contributions
M.R.: data curation, formal analysis, methodology, investigation, visualization, writing-original draft preparation. M.P.: data curation, conceptualization, investigation, visualization, writing-original draft preparation, writing—reviewing and editing. H.R.: visualization, conceptualization, validation, resources, supervision, writing—reviewing and editing. F.Y.: visualization, validation, resources, writing—reviewing and editing. M.N-N.: visualization, validation, writing—reviewing and editing. A.R.: visualization, validation, resources, supervision, writing—reviewing and editing. L.F.R.F.: visualization, validation, writing—reviewing and editing.
Corresponding authors
Ethics declarations
Ethical Approval
None.
Consent to Participate
All authors have given their consent to participate in the study.
Consent for Publication
All authors have given their consent for publication.
Competing Interests
The authors declare no competing interests.
Research Involving Humans and Animals
In our Research, no tests involving Animals or Humans were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Chitosan-based nanomaterials enhance cancer therapy.
• Drug delivery system: chitosan, graphene oxide, agarose, and montmorillonite nanoparticles.
• NC encapsulation with CUR improves breast cancer treatment.
• Results confirm cytotoxicity, pH sensitivity, and sustained drug release against MCF7 cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajaei, M., Pourmadadi, M., Rashedi, H. et al. Improving Curcumin Constraints with pH-Responsive Chitosan Based Graphene Oxide/Montmorillonite Nanohybrid Modified Agarose in Breast Cancer Therapy. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01385-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01385-1