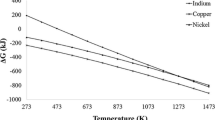
Abstract
Spherical copper powders for additive manufacturing are presently made via gas atomization process which incurs high cost and energy. Present study brings in a novel method for synthesis of spherical copper powders through carbothermic reduction of copper oxides. In this process, fine graphite particles are employed to act as reducing agent as well as spherodizing aid. Effect of graphite (3.5–14 wt%) content on formation of spherical copper powders and effect of temperature (850–1050 °C) and time of reaction (30–240 min) on purity, particle size, density, morphology and flowability of powders are studied. Copper particles with spherical morphology, purity 99 wt% Cu, Oxygen (total) 0.07 wt%, mean particle size 78 µm, apparent density 4.83 g/cc, tap density 5.2 g/cc and flowability 18 s/50 g are obtained at optimized graphite additions of 10.5 wt% and process parameters of 1050 °C and 240 min.








Similar content being viewed by others
Abbreviations
- XRD:
-
X-ray diffraction
- SEM:
-
Scanning electron microscopy
- EDS:
-
Energy-dispersive spectroscopy
- AD:
-
Apparent density
- TD:
-
Tap density
References
Tran T Q, Chinnappan A, Lee J K Y, Loc N H, Tran L T, Wang G, et al., Metals (2019). https://doi.org/10.3390/met9070756
Romano T, Vedani M, Additive Manufacturing of Pure Copper: Technologies and Applications, Copper - From the Mineral to the Final Application [Working Title], (2022) https://doi.org/10.5772/intechopen.107233.
Naboichenko S S, Powder Metall Met Ceram 21 (1982) 843–846. https://doi.org/10.1007/BF00805471
Jhajharia R, Jain D, Sengar A, Goyal A, and Soni P R, Powder Technol 301 (2016) 10–15. https://doi.org/10.1016/j.powtec.2016.05.031
Pease R N, and Taylor H S, J Am Chem Soc 43 (1921) 2179–2188. https://doi.org/10.1021/ja01443a007
Bond W D, J Phys Chem 66 (1962) 1573–1577. https://doi.org/10.1021/j100815a002
Fleisch T H, and Mains G J, Appl Surf Sci 10 (1982) 51–62. https://doi.org/10.1016/0378-5963(82)90134-9
Kiyoshi T, and Toru T, ISIJ Int 9 (1962) 102–108. https://doi.org/10.2497/jjspm.9.102
Sarkisyan N S, Nichiporenko O S, and Kol’chinskii M Z, Powder Metall Met Ceram 30 (1991) 432–435. https://doi.org/10.1007/BF00793675
Sasha A C S, Jordi P, Ricardo H, and Maria D R, Powder Metall 63 (2020) 142–148. https://doi.org/10.1080/00325899.2020.1724431
Sinha A, and Sharma B P, Mater Res Bull 37 (2002) 407–416. https://doi.org/10.1016/S0025-5408(01)00819-4
Songping W, and Shuyuan M, Mater Lett 60 (2006) 2438–2442. https://doi.org/10.1016/j.matlet.2004.08.051
Yoon D K, Kang S L, Eun K Y, and Kim Y, Mater Sci Forum (2007). https://doi.org/10.4028/www.scientific.net/MSF.534-536.109
Wills F, and Clugston E J, J Electrochem Soc 106 (1959) 362. https://doi.org/10.1149/1.2427348
Wang M, Wang Z, and Guo Z, Trans Nonferrous Met Soc China 20 (2010) 1154–1160. https://doi.org/10.1016/S1003-6326(09)60271-5
Seku K, Ganapuram B R, Pejjai B, Kotu G M, and Narasimha G, Int J Nano Dimens 9 (2018) 7–14.
Gunter J, Products, In: Konrad JAK, editor. Copper its Trade, Manufacture, Use and Environmental status, ASM International, United States of America, 1999, 177 pp, Paperback ISBN: 0-87170-656-3.
Neikov O D, Naboychenko S S, and Murashova I B, Handb Non-Ferr Metal Powders (2019). https://doi.org/10.1016/b978-0-08-100543-9.00019-1
Copper & Copper alloy Powders for future technical applications, https://www.schlenk.com/fileadmin/editorsCMS/Medien/02_Maerkte_und_Produkte/pdf/Werkstoffindustrie__BU_MM_/Copper_und_Copper_alloy_powders_12_20.pdf, accessed 04–11–2023
Spherical Copper Powder, https://www.attelements.com/additive-manufacturing-3d-printing-materials/spherical-copper-powder.html, Accessed 04-11-2023
MET Cu-OF-technical data sheet, https://metalpine.at/knowsystem/met-cu-of-technical-data-sheet-210, Accessed 04-11-2023
KME copper powder-Material data sheet, https://www.kme-archiv.com/fileadmin/DOWNLOADCENTER/SPECIAL%20DIVISION/3%20Extruded%20%26%20Drawn/7_KME_POWDER/KME_Powder.pdf, Accessed 04-11-2023
Atomized Copper Powder, http://www.cnpcpowder.com/uploads/soft/160525/AtomizedCopperPowder-CNPCPOWDER.pdf, Accessed 04-11-2023
Kobayashi N, Kawakami Y, Kamada K, Li J G, Ye R, Watanabe T, et al., Trans Mater Res Soc Jpn 31 (2007) 139–142. https://doi.org/10.14723/tmrsj.32.139
Kobayashi N, Kawakami Y, Kamada K, Li J G, Ye R, Watanabe T, et al., Thin Solid Films 516 (2008) 4402–4406. https://doi.org/10.1016/j.tsf.2007.10.064
Sista K S, Moon A P, Sinha G R, Pirjade B M, and Dwarapudi S, Powder Technol 400 (2022) 1–14. https://doi.org/10.1016/j.powtec.2022.117225
Sleptsova N P K, and Ivashchenko A N, Powder Metall Met Ceram 4 (1965) 871–876. https://doi.org/10.1007/BF00773688
Tang S, Cheng Z, Lei C, Huang H, Preparation method for superfine low-oxygen-content spherical copper powder, Patent, CN104874806A (2017). https://patents.google.com/patent/CN104874806A/en.
Tang S, Cheng Z, Lei C, Huang H, Manufacturing method of micron and nanometer metal spherical powder, Patent, CN104259469A (2017). https://patents.google.com/patent/CN104259469A/en.
Wang X, Hanson J C, Frenkel A I, Kim J Y, and Rodriguez J A, J Phys Chem B 108 (2004) 13667–13673. https://doi.org/10.1021/jp040366o
Liu L, Zhnag T J, and Cui K, J Mater Res 14 (1999) 4062–4069. https://doi.org/10.1557/JMR.1999.0548
Lebukhova N V, and Karpovich N F, Inorganic Materials 44 (2008) 890–893. https://doi.org/10.1134/s0020168508080207
Mondal K, Lorethova H, Hippo E, Wiltowski T, and Lalvani S B, Fuel ProcessTechnol 86 (2004) 33–47. https://doi.org/10.1016/j.fuproc.2003.12.009
Mechachti S, Benchiheub O, Serrai S, and Shalabi M E H, Int J Eng Res 4 (2013) 1467–1472.
Goldstein E A, and Mitchell R E, Proc Combust Inst 33 (2011) 2803–2810. https://doi.org/10.1016/j.proci.2010.06.080
Pavlović M G, Pavlović L J, Ivanović E R, Radmilović V, and Popov K I, J Serb Chem Soc 66 (2001) 923–933. https://doi.org/10.2298/JSC0112923P
John WC, Brian HP, Bulk Properties of Powders, In: Prasan KSJ, W. Newkirk., editor. ASM Handbook, Volume 7: Powder Metallurgy, ASM International 2015, 668–77 pp, ISBN: 978-1-62708-089-3.
Sista K S, Dwarapudi S, Kumar D, and Sinha G R, ISIJ Int 60 (2020) 1669–1674. https://doi.org/10.2355/isijinternational.ISIJINT-2019-737
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report there are no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sista, K.S., Moon, A.P., Agrawal, S. et al. Synthesis of Spherical Copper Powders by Reduction Process. Trans Indian Inst Met 77, 889–896 (2024). https://doi.org/10.1007/s12666-023-03199-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-023-03199-3