Abstract
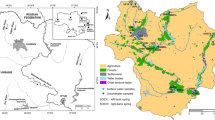
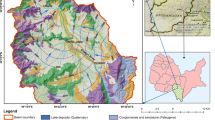
A multi approach methodology using hydrochemistry, environmental stable isotopes and multivariate statistical analysis, were carried out to identify the sources and distributions of groundwater nitrate pollution. Based on the spatial distribution of nitrate concentrations, nitrate pollution occurs mainly in the central part of the study area. Thus, the irrigated areas are likely to be the most affected by this pollution. More than 51.8 % of the sampled wells exceed the maximum contaminant level of 50 mg/L for drinking water. High nitrate levels are associated with isotopic enrichment of δ18O values, clearly indicating that evaporation of irrigation water during infiltration and groundwater contamination. δ18O–NO3 − trends suggest isotopic distinct, non-point source origins which vary spatially and temporally, due to different degrees of evaporation, irrigation return flow and the intensive use of mineral and organic fertilizers and wastewater seepage. However, the anomalies of nitrates in the vicinities of Kabbass tannery are considered as point pollution by dumping of industrial wastewater into drainage network, without treatment. PCA showed the existence of four significant PCs that explain 70 % of the variance. FI represents the nitrates pollution of groundwater. FII exhibits the mineralization processes by interaction between groundwater and the host rocks. FIII and FIV indicate anthropogenic contamination by heavy metal. The proposed approaches have successfully illustrated and assessed the groundwater nitrate pollution.













Similar content being viewed by others
References
Abdi H, Williams LJ (2010) Principal component analysis, Wiley interdisciplinary reviews: computational statistics, 2 (in Press)
Abou Zakhem A, Hafez R (2001) General hydro-isotopic study of direct infiltration and evaporation process through the unsaturated zone in Damascus Oasis, Syria, IAEA-TECDOC-1246, Vienna, pp 131–170
Abou Zakhem A, Hafez R (2009) Chemical and isotopic study of pollutants transport through unsaturated zone in Damascus Oasis (Syria). IAEA-TECDOC-1618, pp 169–193
Abou Zakhem A, Hafez R (2010) Climatic factors controlling chemical and isotopic characteristics of precipitation in Syria. Hydrol Process 24(18):2641–2654
Abou Zakhem A, Hafez R (2014) Heavy metal pollution index for groundwater quality assessment in Damascus Oasis, Syria. Environ Earth Sci. doi:10.1007/s12665-014-3882-5
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
Aghzar N, Berdai H, Bellouti A, Soudi B (2002) Groundwater Nitrate Pollution in Tadla (Morocco). Rev Sci Eau 15:492–495
Ann Soule, Hg L (2011) Groundwater quality monitoring in the shallow aquifer near Sequim, Clallam County, WA, Clallam County Marine Resources Committee CZM 310 Grant no. G10-00003 Task 3.2
Antonakos A, Lambrakis N (2000) Hyhdrodynamic characteristics and nitrate propagation in Sparta aquifer. Wat Res 34(16):3977–3986
Appelo CAJ, Postma D (1993) Geochemistry, groundwater and pollution. In: Balkema AA (ed) Rotterdam, p 535
Berdai H, Aghzar N, Cherkaoui FZ, Soudi B (2002) Azote minéral résiduel et son évolution pendant l’été en fonction du précédent cultural en climat méditerranéen. EGS 9:7–23
Cidu R, Biddau R, Fanfani L (2009) Impact of past mining activity on the quality of groundwater in SW Sardinia (Italy). J Geochem Explor 100:125–132
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York, p 311
Cloutier V, Lefebvre RR, Therrien M, Savard M (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353:294–313
Colleen S (1993) The effect of nitrate, nitrite and N-nitrosocompounds on human health. Review Vet Hum Toxicol 35:521–538
Cosserat M, Decau J, Patacq-Crontzet H, Pujol B (1990) Fertigation in coarse-textured soil consequences on production and nitrate pollution. In: Calvet R (ed), Nitrate-Agric.-Eau, INRA, Paris, France, pp 257–262
Craig H (1961) Isotopic variations in meteoric water. Science 133:1702
Criss RE, Davisson ML (1996) Isotope imaging of surface water/groundwater interactions, Sacramento Valley, California. J Hydrol 178:205–222
Davis JC (1986) Statistics and data analysis in geology. Wiley, New York
Davisson ML, Criss RE (1993) Stable isotope imaging of a dynamic groundwater sys. in the south-western Sacramento Valley, California (USA). J Hydrol 144:213–246
Deutsch WJ (1997) Groundwater geochemistry: fundamentals and applications to contamination. Lewis, New York, p 221
Epstein S, Mayeda TK (1953) Variations of the 18O/16O ratio innatural waters. Geochim Cosmochim Acta 4:213–224
Gat JR, Carmi I (1970) Evolution of the isotopic composition of atmospheric water in Mediterranean Sea Area. J Geophys Res pp 3039–3048
Hamzaoui-Azaza F, Bouhlila R, Gueddari M (2009) Geochemistry of fluoride and major ion in the groundwater samples of triassic aquifer (South Eastern Tunisia), through multivariate and hydrochemical techniques. J App Sci Res 5(11):1941–1951
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of ground water composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res 34:807–816
Homsi M, Bouni M, Kurdi A (1989) Damascus basin climate and its role in economical plans and the climatic changes. Nat Meteorol Rep. p 35
Jakson JE (1991) A user’s guide to principal components analyses. Wiley, New York
Kattan Z (2006) Characterization of surface water and groundwater in the Damascus Ghotta basin: hydrochemical and environmental isotopes approaches. Environ Geol 51:173–201
Kim JG, Chon CM, Lee JS (2004) Effect of structure and texture on infiltration flow pattern during flood irrigation. Environ Geol 46:962–969
Lake IR, Lovett AA, Hiscock KM, Betson M, Foley A, Sünnenberg G, Evers S, Fletcher S (2003) Evaluating factors influencing groundwater vulnerability to nitrate pollution: developing the potential of GIS. J Environ Manage 68:315–328
Liu A, Ming J, Ankumah RO (2005) Nitrate contamination in private wells in rural Alabama, United States. Sci Total Environ 346:112–120
Mcclain M, Richey J, Pimentel T (1994) Groundwater nitrogen dynamics at the terrestrial-lotic interface of a small catchment in the Central Amazon Basin. Biogeochemistry 27:113–127
Meglen RR (1992) Examining large databases: a chemo-metric approach using principal component analysis. Mar Chem 39:217–237
Mikkelsen SA (1992) Current nitrate research in Denmark-background and practical application, nitrate and farming systems. Asp Appl Biol 30:29–44
Ministry of Environment (1994) Drinking water quality standards. Higher council for environment and safety. Damascus, Syria (Arabic)
Ministry of Housing (2000) Adra treatment plant, the Ministry of housing documents, Damascus
Montero Alvarez A, Estévez Alvarez JR, Padilla Alvarez R (2007) Heavy metal analysis of rain waters: a comparison of TXRF and ASV analytical capabilities. J Radioanal Nucl Chem 273(2):427–433
Nir (1967) development of isotope methods applied to groundwater hydrology. In: Proceeding of a symposium on isotope technique in hydrological cycle. Am Geophys. Union Monogr Series, no 11
Pixie AH, Dennis RH (1995) Effects of agric. on groundwater quality in five regions of the United States. Gr Water 33:217–226
Ponikarov VP (1966) Explanatory notes on the geological map of Syria, scale 1/200.000, sheet VII (Damascus) “Techno-export”
Rao NS (2006) Nitrate pollution and its distribution in the groundwater of Srikakulam district, Andhra Pradesh, India. Environ Geol 51:631–645
Rivett MO, Buss SR, Morgan P, Smith JWN, Bemment CD (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res 42:4215–4232
Selkhozpromexport (1986) Water resources use in Barada and Awage basins for irrigation of crops, vol II, book 2, USSR, Moscow. p 484
Shimojima E, Sharma ML (1995) The influence of pore water velocity on transport of sorptive and non sorptive chemical through an unsaturated sand. J Hydrol 164:239–261
Sonthalia P, McGaw E, Show Y, Greg M, Swain GM (2004) Metal ion analysis in contaminated water samples using anodic stripping voltammetry and a nanocrystalline diamond thin-film electrode. Anal Chim Acta 522(2004):35–44
Soumi G, Chayeb R (1989) Water need and plant irrigation techniques. Rep. UNDP, FAO, SYR/86/015, agriculture ministry. p 33
Stamatis G, Parpodis K, Filintas A, Zagana E (2011) Groundwater quality, nitrate pollution and irrigation environmental management in the Neogene sediments of an agricultural region in central Thessaly (Greece). Environ Earth Sci 64:1081–1105
Subyani AM (2005) Hydrochemical identification and salinity problem of ground-water in Wadi Yalamlam basin, Western Saudi Arabia. J Arid Environ 60:53–66
Tagma T, Hsissou Y, Bouchaou L, Bouragba L, Boutaleb S (2009) Groundwater nitrate pollution in Souss-Massa basin (south-west Morocco). Afr J Environ Sci Technol 3(10):301–309
Thorburn P, Biggs J, Weier K, Keating B (2003) Nitrate in groundwater of intensive agricultural areas in coastal Northeastern Australia. Agric Ecosyst Environ 94:49–58
Vinten AJA, Vivian BJ, Wright F, Howard RS (1994) A comparative study of nitrate leaching from soils of differing textures under similar climatic and cropping conditions. J Hydrol 159:197–213
Wakida F, Lerner D (2002) Nitrate leaching from construction sites to groundwater in Nottingham, UK, urban area. Water Sci Technol 45:243–248
WHO (2004) Guidelines for drinking water quality. Vol 1 recommendations, 3rd ed. WHO, Geneva p 540
Widory D, Kloppmann W, Chery L, Bonnin J, Rochdi H, Guinamant JL (2004) Nitrate in groundwater: an isotopic multi-tracer approach. J Contam Hydrol 72:165–188
World Bank Group (1998) Pollution prevention and abatement handbook, World Bank
Acknowledgments
The authors would like to gratefully acknowledge Prof. Dr. I. Othman, director general of AECS, for his guidance and support. Special thanks to Dr. K. M. Kulkarni, technical officer of the CRP No. SYR.11517 (IAEA). Thanks to AECS laboratory team for chemical and isotopic analysis. Thanks to B. Katta for mapping work. Finally, thanks to the colleagues in Geology Department (AECS). This study was done under the framework of CRP project no. SYR.11517 in cooperation with International Atomic Energy Agency (IAEA) entitled “Chemical and Isotopic Study of Pollutants Transport through Unsaturated Zone in Damascus Oasis (Syria)” (IAEA-TECDOC-1618, 2009).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abou Zakhem, B., Hafez, R. Hydrochemical, isotopic and statistical characteristics of groundwater nitrate pollution in Damascus Oasis (Syria). Environ Earth Sci 74, 2781–2797 (2015). https://doi.org/10.1007/s12665-015-4258-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4258-1