Abstract
Maintaining an optimal concentration of nutrients in photobioreactors (PBRs) is a key issue for their optimal design and operation. In this study, a numerical investigation was conducted to quantify the dissolution of KNO3 and Na2HPO4 inside a thin-layer cascade (TLC) reactor and determine its consequential effect on the reactor performance for algal cultivation. A computational fluid dynamics (CFD) model based on Euler–Euler approach was used to investigate nutrient mixing in TLC and evaluate the effect of flow and geometric properties of the reactor. A wide range of pertinent parameters such as channel width, channel depth, mass flow rate and nutrient particle size were considered. Nutrient concentration plots, nutrient mixing in terms of mass transfer coefficient, and solid hold-up in the reactor were established. The nutrient dissolution improved in the reactor with small dimensions operating at high mass flow rates and was inversely related to the nutrient particle size; that is, small particle results in increased nutrient mixing due to the enlarged interfacial area.
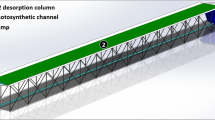
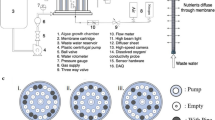
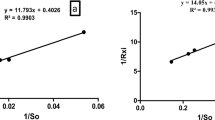
Graphical Abstract














Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- \(AR\) :
-
Aspect ratio
- \(D\) :
-
Diffusion coefficient (m2/s)
- \(d\) :
-
Water depth (m)
- \({\varvec{u}}\) :
-
Velocity vector (m/s)
- \(W\) :
-
Channel width (m)
- \(A\) :
-
Cross sectional area (m2)
- \(L\) :
-
Length (m)
- \({D}_{h}\) :
-
Hydraulic diameter (m)
- \(\mathrm{a}\) :
-
Interfacial area (1/m)
- \({c}_{\mathrm{c}}\) :
-
Dissolved particle concentration in liquid (mol/m3)
- \({c}_{a}\) :
-
Initial particle concentration in liquid (mol/m3)
- \({c}_{d}\) :
-
Mass fraction
- \(Fr\) :
-
Froude number (1)
- \(Mv\) :
-
Density number (1)
- \({V}_{l}\) :
-
Liquid velocity (m/s)
- \({d}_{p}\) :
-
Solid particle diameter (m)
- \({D}_{md}\) :
-
Dispersion coefficient (m2/s)
- \(Sc\) :
-
Schmidt number
- g\(,\) :
-
Gravitational vector (m/s2)
- \(Re\) :
-
Reynolds number
- \({k}_{sl}\) :
-
Solid liquid mass transfer coefficient (m/s)
- \(M\) :
-
Molecular weight (kg/mol)
- \({m}_{dc}\) :
-
Mass transfer rate (kg/(m3 s))
- \(p\) :
-
Pressure (Pa)
- \({\mathbf{u}}_{\mathrm{slip}}\) :
-
Slip velocity vector (m/s)
- \(n\) :
-
Number of particle per volume (1/m3)
- \(k\) :
-
Turbulent kinetic energy (m2/s2)
- \(\mu \) :
-
Dynamic viscosity (Pa s)
- \({\mu }_{T}\) :
-
Turbulent viscosity (Pa s)
- \({\sigma }_{k}\) :
-
Prandtl number for kinetic energy
- \({\sigma }_{\varepsilon }\) :
-
Prandtl number for dissipation rate
- \(\nabla \) :
-
Gradient operator
- \(\varphi \) :
-
Volume fraction/hold-up
- \(\rho \) :
-
Water density (kg/m3)
- \(\varepsilon \) :
-
Turbulent energy dissipation rate (m2/s3)
- \(p\) :
-
Particle
- \(s\) :
-
Solid phase
- \(d\) :
-
Dispersed phase
- \(c\) :
-
Continuous phase
- \(l\) :
-
Liquid phase
References
Mostert, E.S., Grobbelaar, J.U.: The influence of nitrogen and phosphorus on algal growth and quality in outdoor mass algal cultures. Biomass 13(4), 219–233 (1987). https://doi.org/10.1016/0144-4565(87)90061-8
Grobbelaar, J.U., Soeder, C.J., Stengel, E.: Modeling algal productivity in large outdoor cultures and waste treatment systems. Biomass 21(4), 297–314 (1990). https://doi.org/10.1016/0144-4565(90)90079-Y
Morales-Amaral, M.M., Gómez-Serrano, C., Acién, F.G., Fernández-Sevilla, J.M., Molina-Grima, E.: Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion as the sole nutrient source. Algal Res. 12, 99–108 (2015). https://doi.org/10.1016/j.algal.2015.08.020
Juneja, A., Ceballos, R.M., Murthy, G.S.: Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6(9), 4607–4638 (2013). https://doi.org/10.3390/en6094607
Daliry, S., Hallajisani, A., Mohammadi, R.J., Nouri, H., Golzary, A.: Investigation of optimal condition for Chlorella vulgaris microalgae growth. Glob. J. Environ. Sci. Manag. 3(2), 217–230 (2017). https://doi.org/10.22034/gjesm.2017.03.02.010
Cromar, N.J., Fallowfield, H.J.: Effect of nutrient loading and retention time on performance of high rate algal ponds. J. Appl. Phycol. 9(4), 301–309 (1997). https://doi.org/10.1023/A:1007917610508
Goldman, J.C.: Physiological processes, nutrient availability, and the concept of relative growth rate in marine phytoplankton ecology. In: Falkowski, P.G. (ed.) Primary productivity in the sea, pp. 179–194. Springer, Boston (1980)
Borowitzka, M.A., Huisman, J.M., Osborn, A.: Culture of the astaxanthin-producing green alga Haematococcus pluvialis 1. Effects of nutrients on growth and cell type. J. Appl. Phycol. 3(4), 295–304 (1991). https://doi.org/10.1007/BF02392882
Xin, L., Hong-Ying, H., Ke, G., Ying-Xue, S.: Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 101(14), 5494–5500 (2010). https://doi.org/10.1016/j.biortech.2010.02.016
Khozin-Goldberg, I., Cohen, Z.: The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67(7), 696–701 (2006). https://doi.org/10.1016/j.phytochem.2006.01.010
Cakmak, T., Angun, P., Demiray, Y.E., Ozkan, A.D., Elibol, Z., Tekinay, T.: Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol. Bioeng. 109(8), 1947–1957 (2012). https://doi.org/10.1002/bit.24474
Goldman, J.C.: Outdoor algal mass cultures—II. Photosynthetic yield limitations. Water Res. 13(2), 119–136 (1979). https://doi.org/10.1016/0043-1354(79)90083-6
Richmond, A., Grobbelaar, J.U.: Factors affecting the output rate of Spirulina platensis with reference to mass cultivation. Biomass 10(4), 253–264 (1986). https://doi.org/10.1016/0144-4565(86)90002-8
Ruiz-Marin, A., Mendoza-Espinosa, L.G., Stephenson, T.: Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 101(1), 58–64 (2010). https://doi.org/10.1016/j.biortech.2009.02.076
Marchetti, A., et al.: Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature 457(7228), 467–470 (2009). https://doi.org/10.1038/nature07539
Grobbelaar, J.U., Kroon, B.M.A., Burger-Wiersma, T., Mur, L.R.: Influence of medium frequency light/dark cycles of equal duration on the photosynthesis and respiration of Chlorella pyrenoidosa. Hydrobiologia 238(1), 53–62 (1992). https://doi.org/10.1007/BF00048773
Borowitzka, M.A.: Commercial production of microalgae: ponds, tanks, tubes and fermenters. J. Biotechnol. 70(1), 313–321 (1999). https://doi.org/10.1016/S0168-1656(99)00083-8
Akhtar, S., Ali, H., Park, C.W.: Complete evaluation of cell mixing and hydrodynamic performance of thin-layer cascade reactor. Appl. Sci. (2020). https://doi.org/10.3390/app10030746
Pruvost, J., Pottier, L., Legrand, J.: Numerical investigation of hydrodynamic and mixing conditions in a torus photobioreactor. Chem. Eng. Sci. 61(14), 4476–4489 (2006). https://doi.org/10.1016/j.ces.2006.02.027
Zheng, H., et al.: Balancing carbon/nitrogen ratio to improve nutrients removal and algal biomass production in piggery and brewery wastewaters. Bioresour. Technol. 249, 479–486 (2018). https://doi.org/10.1016/j.biortech.2017.10.057
Masojídek, J., Kopecký, J., Giannelli, L., Torzillo, G.: Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J. Ind. Microbiol. Biotechnol. 38(2), 307–317 (2011). https://doi.org/10.1007/s10295-010-0774-x
Grobbelaar, J.U.: Factors governing algal growth in photobioreactors: the ‘open’ versus ‘closed’ debate. J. Appl. Phycol. 21(5), 489–492 (2009). https://doi.org/10.1007/s10811-008-9365-x
Eustance, E., Wray, J.T., Badvipour, S., Sommerfeld, M.R.: The effects of cultivation depth, areal density, and nutrient level on lipid accumulation of Scenedesmus acutus in outdoor raceway ponds. J. Appl. Phycol. 28(3), 1459–1469 (2016). https://doi.org/10.1007/s10811-015-0709-z
Chisti, Y.: Biodiesel from microalgae. Biotechnol. Adv. 25(3), 294–306 (2007). https://doi.org/10.1016/j.biotechadv.2007.02.001
Conley, D.J., et al.: Ecology-Controlling eutrophication: nitrogen and phosphorus. Science (80-) 323(5917), 1014–1015 (2009). https://doi.org/10.1126/science.1167755
Ali, H., Zhang, D., Wagner, J.L., Park, C.W.: Two-phase flow modeling of solid dissolution in liquid for nutrient mixing improvement in algal raceway ponds. Energies 11(4), 1–21 (2018). https://doi.org/10.3390/en11040899
G. Shelef and C. J. Soeder, Algae biomass: production and use. (1980).
Kalaga, D.V., Dhar, A., Dalvi, S.V., Joshi, J.B.: Particle–liquid mass transfer in solid–liquid fluidized beds. Chem. Eng. J. 245, 323–341 (2014). https://doi.org/10.1016/j.cej.2014.02.038
Tang, C., Liu, M., Li, Y.: Experimental investigation of hydrodynamics of liquid–solid mini-fluidized beds. Particuology 27, 102–109 (2016). https://doi.org/10.1016/j.partic.2015.03.011
Palkar, R.R., Shilapuram, V.: Development of a model for the prediction of hydrodynamics of a liquid–solid circulating fluidized beds: a full factorial design approach. Powder Technol. 280, 103–112 (2015). https://doi.org/10.1016/j.powtec.2015.04.045
Ballesteros, R.L., Riba, J.P., Couderc, J.P.: Dissolution of non spherical particles in solid–liquid fluidization. Chem. Eng. Sci. 37(11), 1639–1644 (1982). https://doi.org/10.1016/0009-2509(82)80034-1
Yang, Z., del Ninno, M., Wen, Z., Hu, H.: An experimental investigation on the multiphase flows and turbulent mixing in a flat-panel photobioreactor for algae cultivation. J. Appl. Phycol. 26(5), 2097–2107 (2014). https://doi.org/10.1007/s10811-014-0239-0
Li, Y., Che, D., Liu, Y.: CFD simulation of hydrodynamic characteristics in a multiple-spouted bed. Chem. Eng. Sci. 80, 365–379 (2012). https://doi.org/10.1016/j.ces.2012.06.003
Chiesa, M., Mathiesen, V., Melheim, J.A., Halvorsen, B.: Numerical simulation of particulate flow by the Eulerian–Lagrangian and the Eulerian–Eulerian approach with application to a fluidized bed. Comput. Chem. Eng. 29(2), 291–304 (2005). https://doi.org/10.1016/j.compchemeng.2004.09.002
Enwald, H., Peirano, E., Almstedt, A.-E.: Eulerian two-phase flow theory applied to fluidization. Int. J. Multiph. Flow 22, 21–66 (1996). https://doi.org/10.1016/S0301-9322(96)90004-X
Cornelissen, J.T., Taghipour, F., Escudié, R., Ellis, N., Grace, J.R.: CFD modelling of a liquid–solid fluidized bed. Chem. Eng. Sci. 62(22), 6334–6348 (2007). https://doi.org/10.1016/j.ces.2007.07.014
Yang, N., Wang, W., Ge, W., Li, J.: CFD simulation of concurrent-up gas–solid flow in circulating fluidized beds with structure-dependent drag coefficient. Chem. Eng. J. 96(1), 71–80 (2003). https://doi.org/10.1016/j.cej.2003.08.006
Apel, A.C., et al.: Open thin-layer cascade reactors for saline microalgae production evaluated in a physically simulated Mediterranean summer climate. Algal Res. 25(March), 381–390 (2017). https://doi.org/10.1016/j.algal.2017.06.004
Chow, V.T.: Open-channel hydraulics. In: Perry, B. (ed.) Open-Channel Hydraulics. McGraw-Hill, New York (1959)
Wilcox, D.C.: Turbulence Modeling for CFD. DCW industries, La Canada (1998)
Silva, R., Cotas, C., Garcia, F.A.P., Faia, P.M., Rasteiro, M.G.: Particle distribution studies in highly concentrated solid–liquid flows in pipe using the mixture model. Procedia Eng. 102, 1016–1025 (2015). https://doi.org/10.1016/j.proeng.2015.01.224
Ali, H., Cheema, T.A., Park, C.W.: Numerical modeling of two-phase bubbly flow mixing with mass transport in an effective microorganism odor removing system. J. Chem. Technol. Biotechnol. 91(4), 1012–1022 (2016). https://doi.org/10.1002/jctb.4674
Ali, H., Cheema, T.A., Yoon, H.S., Do, Y., Park, C.W.: Numerical prediction of algae cell mixing feature in raceway ponds using particle tracing methods. Biotechnol. Bioeng. 112(2), 297–307 (2015). https://doi.org/10.1002/bit.25443
Ali, H., Cheema, T.A., Park, C.W.: Effect of paddle-wheel pulsating velocity on the hydrodynamic performance of high-rate algal ponds. J. Energy Eng. 141(4), 4014039 (2015). https://doi.org/10.1061/(ASCE)EY.1943-7897.0000219
Weissman, J.C., Goebel, R.P., Benemann, J.R.: Photobioreactor design: mixing, carbon utilization, and oxygen accumulation. Biotechnol. Bioeng. 31(4), 336–344 (1988). https://doi.org/10.1002/bit.260310409
Grobbelaar, J.U., Nedbal, L., Tichy, L., Setlik, I.: Variation in some photosynthetic characteristics of microalgae cultured in outdoor thin-layered sloping reactors. J. Appl. Phycol. 7(2), 175–184 (1995). https://doi.org/10.1007/BF00693065
Akhtar, S., Ali, H., Park, C.W.: Effective prediction of monthly heat transfer characteristics in a thin-layer cascade reactor subjected to outdoor conditions. J. Chem. 2022, 1–16 (2022). https://doi.org/10.1155/2022/3209051
Camacho, F.G., Gomez, A.C., Sobczuk, T.M., Grima, E.M.: Effects of mechanical and hydrodynamic stress in agitated, sparged cultures of Porphyridium cruentum. Process Biochem. 35(9), 1045–1050 (2000). https://doi.org/10.1016/S0032-9592(00)00138-2
Quiroz-Arita, C., et al.: A dynamic thermal algal growth model for pilot-scale open-channel raceways. Bioresour. Technol. Rep. 10, 100405 (2020). https://doi.org/10.1016/j.biteb.2020.100405
Thomas, W.H., Gibson, C.H.: Effects of small-scale turbulence on microalgae. J. Appl. Phycol. 2(1), 71–77 (1990). https://doi.org/10.1007/BF02179771
Grobbelaar, J.U.: Turbulence in mass algal cultures and the role of light/dark fluctuations. J. Appl. Phycol. 6(3), 331–335 (1994). https://doi.org/10.1007/BF02181947
Chen, C.-Y., Yeh, K.-L., Aisyah, R., Lee, D.-J., Chang, J.-S.: Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour. Technol. 102(1), 71–81 (2011). https://doi.org/10.1016/j.biortech.2010.06.159
Acién Fernández, F.G., García Camacho, F., Chisti, Y.: Photobioreactors: light regime, mass transfer, and scaleup. In: Osinga, R., Tramper, J., Burgess, J.G., Wijffels, R.H. (eds.) Marine Bioprocess Engineering, pp. 231–247. Amsterdam, Elsevier (1999)
Acknowledgements
This study is supported by the National Research Foundation of Korea and funded by the Korean government (MSIP, Grant Nos. 2020R1A2B5B02002512 and 2020R1A4A1018652).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akhtar, S., Memon, S.A., Siddiqa, S. et al. Numerical Investigation of Solid–Liquid Dissolution for Nutrient Mixing Improvement in a Thin-Layer Cascade System. Waste Biomass Valor 15, 771–785 (2024). https://doi.org/10.1007/s12649-023-02180-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02180-x