Abstract
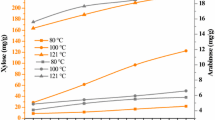
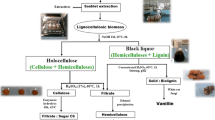
The palm press fiber, resulting from the extraction of oil from the fruit of the oil palm (Elaeis guineensis) is an abundant agro-industrial co-product with a potential for development of biorefineries. This study evaluated the use of the hemicellulose fraction contained in the palm press fiber as a source of sugars for the production of bioethanol by Scheffersomyces stipitis. The optimal condition for hemicellulose hydrolysis, determined by response surface methodology, utilized 30% of dry biomass in 5% H2SO4 at 121 °C for 60 min, and resulted in removal of 88.4% of this polysaccharide. The soluble fraction recovered after the acid pretreatment, called hemicellulosic hydrolyzate, contained 83 g L−1 of reducing sugars. The hydrolyzate also contained 12 g L−1 of acetic acid, 489 mg L−1 of furfural and 46 mg L−1 of 5-hydroxymethylfurfural. The detoxification of the hydrolyzate with activated charcoal, overliming and a combination thereof was evaluated for removal of unwanted byproducts. The best detoxification treatment reduced the concentrations of phenolic compounds and furfural present in the hemicellulosic hydrolyzate by 96% and 99%, respectively. S. stipitis NRRLY 7124 and S. stipitis CBS 6054 were tested for the fermentation of the hydrolyzate. The highest yield of ethanol, 0.33 gethanol gsugar −1, was obtained with the NRRLY 7124 strain in the fermentation of the hydrolyzate detoxified by overliming. An estimated production of 12.1 L of ethanol per ton of palm press fiber derived solely from the hemicellulosic fraction was achieved.



Similar content being viewed by others
References
Basiron, Y.: Palm oil production through sustainable plantations. Eur. J. Lipid. Sci. Technol. 109, 289–295 (2007). doi:10.1002/ejlt.200600223
USDA: Oilseeds: world markets and trades. http://www.fas.usda.gov/data/oilseeds-world-markets-and-trade (2016). Accessed 15 Sept 2016
Kurnia, J.C., Jangam, S.V., Akhtar, S., Sasmito, A.P., Mujumdar, A.S.: Advances in biofuel production from oil palm and palm oil processing wastes: a review. Biofuel Res. J. 3, 332–346 (2016). doi:10.18331/BRJ2016.3.1.3
Tan, Y.-A.: By-products of palm oil extraction and refining. Ol. Corps gras Lipid. 13, 9–11 (2006). doi:10.1051/ocl.2006.8888
Riansa-ngawong, W., Prasertsan, P.: Optimization of furfural production from hemicellulose extracted from delignified palm pressed fiber using a two-stage process. Carbohydr. Res. 346, 103–110 (2011). doi:10.1016/j.carres.2010.10.009
Ofori-Boateng, C., Lee, K.T., Saad, B.: A biorefinery concept for simultaneous recovery of cellulosic ethanol and phenolic compounds from oil palm fronds: process optimization. Energy Convers. Manag. 81, 192–200 (2014). doi:10.1016/j.enconman.2014.02.030
Ali, A.A.M., Othman, M.R., Shirai, Y., Hassan, M.A.: Sustainable and integrated palm oil biorefinery concept with value-addition of biomass and zero emission system. J. Clean. Prod. 91, 96–99 (2015). doi:10.1016/j.jclepro.2014.12.030
Abdullah, S.S.S., Shirai, Y., Ali, A.A.M., Mustapha, M., Hassan, M.A.: Case study: Preliminary assessment of integrated palm biomass biorefinery for bioethanol production utilizing non-food sugars from oil palm frond petiole. Energy Convers. Manag. 108, 233–242 (2016). doi:10.1016/j.enconman.2015.11.016
Shinoj, S., Visvanathan, R., Panigrahi, S., Kochubabu, M.: Oil palm fiber (OPF) and its composites: a review. Ind. Crops Prod. 33, 7–22 (2011). doi:10.1016/j.indcrop.2010.09.009
Neoh, B.K., Thang, Y.M., Zain, M.Z.M., Junaidi, A.: Palm pressed fibre oil: a new opportunity for premium hardstock? Int. Food Res. J. 18, 769–773 (2011).
Boonsawang, P., Subkaree, Y., Srinorakutara, T.: Ethanol production from palm pressed fiber by prehydrolysis prior to simultaneous saccharification and fermentation (SSF). Biomass Bioenergy. 40, 127–132 (2012). doi:10.1016/j.biombioe.2012.02.009
Kami Delivand, M., Gnansounou, E.: Life cycle environmental impacts of a prospective palm-based biorefinery in Pará State-Brazil. Bioresour. Technol. 150, 438–446 (2013). doi:10.1016/j.biortech.2013.07.100
Rincón, L.E., Moncada, J., Cardona, C.A.: Analysis of potential technological schemes for the development of oil palm industry in Colombia: a biorefinery point of view. Ind. Crops. Prod. 52, 457–465 (2014). doi:10.1016/j.indcrop.2013.11.004
Zakaria, M.R., Hirata, S., Hassan, M.A.: Combined pretreatment using alkaline hydrothermal and ball milling to enhance enzymatic hydrolysis of oil palm mesocarp fiber. Bioresour. Technol. 169, 236–243 (2014). doi:10.1016/j.biortech.2014.06.095
Gírio, F.M., Fonseca, C., Carvalheiro, F., Duarte, L.C., Marques, S., Bogel-Łukasik, R.: Hemicelluloses for fuel ethanol: a review. Bioresour. Technol. 101, 4775–4800 (2010). doi:10.1016/j.biortech.2010.01.088
Cerveró, J.M., Skovgaard, P.A., Felby, C., Sørensen, H.R., Jørgensen, H.: Enzymatic hydrolysis and fermentation of palm kernel press cake for production of bioethanol. Enzyme Microb. Technol. 46, 177–184 (2010). doi:10.1016/j.enzmictec.2009.10.012
Menon, V., Rao, M.: Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 38, 522–550 (2012). doi:10.1016/j.pecs.2012.02.002
Medina, J.D.C., Woiciechowski, A., Filho, A.Z., Nigam, P.S., Ramos, L.P., Soccol, C.R.: Steam explosion pretreatment of oil palm empty fruit bunches (EFB) using autocatalytic hydrolysis: a biorefinery approach. Bioresour. Technol. 199, 173–180 (2016). doi:10.1016/j.biortech.2015.08.126
Guo, G.-L., Chen, W.-H., Chen, W.-H., Men, L.-C., Hwang, W.-S.: Characterization of dilute acid pretreatment of silvergrass for ethanol production. Bioresour. Technol. 99, 6046–6053 (2008). doi:10.1016/j.biortech.2007.12.047
Kundu, C., Trinh, L.T.P., Lee, H.-J., Lee, J.-W.: Bioethanol production from oxalic acid-pretreated biomass and hemicellulose-rich hydrolysates via a combined detoxification process. Fuel. 161, 129–136 (2015). doi:10.1016/j.fuel.2015.08.045
Chandel, A.K., Antunes, F.A.F., Arruda, P.V., Milessi, T.S.S., Silva, S.S., Almeida Felipe, M. G.: Dilute acid hydrolysis of agro-residues for the depolymerization of hemicellulose: state-of-the-art. In: Silva, S.S., Chandel, A.K. (eds.) D-Xylitol, pp. 39–61. Springer, Berlin (2012). doi:10.1007/978-3-642-31887-0_2
Sun, Z., Shupe, A., Liu, T., Hu, R., Amidon, T.E., Liu, S.: Particle properties of sugar maple hemicellulose hydrolysate and its influence on growth and metabolic behavior of Pichia stipitis. Bioresour. Technol. 102, 2133–2136 (2011). doi:10.1016/j.biortech.2010.08.097
Toquero, C., Bolado, S.: Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour. Technol. 157, 68–76 (2014). doi:10.1016/j.biortech.2014.01.090
Camesasca, L., Ramírez, M.B., Guigou, M., Ferrari, M.D., Lareo, C.: Evaluation of dilute acid and alkaline pretreatments, enzymatic hydrolysis and fermentation of napiergrass for fuel ethanol production. Biomass Bioenergy. 74, 193–201 (2015). doi:10.1016/j.biombioe.2015.01.017
Zakaria, M.R., Hirata, S., Fujimoto, S., Ibrahim, I., Hassan, M.A.: Soluble inhibitors generated during hydrothermal pretreatment of oil palm mesocarp fiber suppressed the catalytic activity of Acremonium cellulase. Bioresour. Technol. 200, 541–547 (2016). doi:10.1016/j.biortech.2015.10.075
Gupta, R., Mehta, G., Chander Kuhad, R.: Fermentation of pentose and hexose sugars from corncob, a low cost feedstock into ethanol. Biomass Bioenergy. 47, 334–341 (2012). doi:10.1016/j.biombioe.2012.09.027
Mateo, S., Roberto, I.C., Sánchez, S., Moya, A.J.: Detoxification of hemicellulosic hydrolyzate from olive tree pruning residue. Ind. Crop. Prod. 49, 196–203 (2013). doi:10.1016/j.indcrop.2013.04.046
Mohagheghi, A., Ruth, M., Schell, D.J.: Conditioning hemicellulose hydrolysates for fermentation: effects of overliming pH on sugar and ethanol yields. Process Biochem. 41, 1806–1811 (2006). doi:10.1016/j.procbio.2006.03.028
Kamal, S.M.M., Mohamad, N.L., Abdullah, A.G.L., Abdullah, N.: Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production. Proced. Food Sci. 1, 908–913 (2011). doi:10.1016/j.profoo.2011.09.137
Chi, Z., Rover, M., Jun, E., Deaton, M., Johnston, P., Brown, R.C., Wen, Z., Jarboe, L.R.: Overliming detoxification of pyrolytic sugar syrup for direct fermentation of levoglucosan to ethanol. Bioresour. Technol. 150, 220–227 (2013). doi:10.1016/j.biortech.2013.09.138
Kurtzman, C.P., Suzuki, M.: Phylogenetic analysis of ascomycete yeasts that form coenzyme Q-9 and the proposal of the new genera Babjeviella, Meyerozyma, Millerozyma, Priceomyces, and Scheffersomyces. Mycoscience. 51, 2–14 (2010). doi:10.1007/S10267-009-0011-5
Gutiérrez-Rivera, B., Ortiz-Muñiz, B., Gómez-Rodríguez, J., Cárdenas-Cágal, A., Domínguez González, J.M., Aguilar-Uscanga, M.G.: Bioethanol production from hydrolyzed sugarcane bagasse supplemented with molasses “B” in a mixed yeast culture. Renew. Energy 74, 399–405 (2015). doi:10.1016/j.renene.2014.08.030
Agbogbo, F.K., Coward-Kelly, G.: Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnol. Lett. 30, 1515–1524 (2008). doi:10.1007/s10529-008-9728-z
Purwadi, R., Niklasson, C., Taherzadeh, M.J.: Kinetic study of detoxification of dilute-acid hydrolyzates by Ca(OH)2. J. Biotechnol. 114, 187–198 (2004). doi:10.1016/j.jbiotec.2004.07.006
Pereira Jr., N.: Intensification of the xylose fermentation process. PhD Thesis. The University of Manchester, Manchester (1991)
Bellido, C., Bolado, S., Coca, M., Lucas, S., González-Benito, G., García-Cubero, M.T.: Effect of inhibitors formed during wheat straw pretreatment on ethanol fermentation by Pichia stipitis. Bioresour. Technol. 102, 10868–10874 (2011). doi:10.1016/j.biortech.2011.08.128
Van Soest, P.J.: Development of a comprehensive system of feed analysis and its application to forages. J. Anim. Sci. 26, 119–128 (1967). doi:10.2134/jas1967.261119x
IAL: Métodos físico-químicos para análise de alimentos. Instituto Adolfo Lutz, São Paulo (2008)
DuBois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956). doi:10.1021/ac60111a017
McCready, R.M., Guggolz, J., Silviera, V., Owens, H.S.: Determination of starch and amylose in vegetables. Anal. Chem. 22, 1156–1158 (1950). doi:10.1021/ac60045a016
Singleton, V.L., Rossi, J.A.: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959). doi:10.1021/ac60147a030
Isarankura-Na-Ayudhya, C., Tantimongcolwat, T., Kongpanpee, T., Prabkate, P., Prachayasittikul, V.: Appropriate technology for the bioconversion of water hyacinth (Eichhornia crassipes) to liquid ethanol future prospects for community strengthening and sustainable development. EXCLI J. 6, 167–176 (2007)
Asadieraghi, M., Daud, W.M.A.W.: In-depth investigation on thermochemical characteristics of palm oil biomasses as potential biofuel sources. J. Anal. App. Pyrolysis. 115, 379–391 (2015). doi:10.1016/j.jaap.2015.08.017
Costa, A.G., Pinheiro, G.C., Pinheiro, F.G.C., Dos Santos, A.B., Santaella, S.T., Leitão, R.C.: Pretreatment strategies to improve anaerobic biodegradability and methane production potential of the palm oil mesocarp fibre. Chem. Eng. J. 230, 158–165 (2013). doi:10.1016/j.cej.2013.06.070
Mendu, V., Harman-Ware, A.E., Crocker, M., Jae, J., Stork, J., Morton, S., Placido, A., Huber, G., DeBolt, S.: Identification and thermochemical analysis of high-lignin feedstocks for biofuel and biochemical production. Biotechnol. Biofuels. 4, 43 (2011). doi:10.1186/1754-6834-4-43
Heipieper, H.J., Weber, F.J., Sikkema, J., Keweloh, H., de Bont, J.A.M.: Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 12, 409–415 (1994). doi:10.1016/0167-7799(94)90029-9
Modig, T., Lidén, G., Taherzadeh, M.J.: Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 363, 769–776 (2002)
Delgenes, J.P., Moletta, R., Navarro, J.M.: Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzyme Microb. Technol. 19, 220–225 (1996). doi:10.1016/0141-0229(95)00237-5
Yücel, H.G., Aksu, Z.: Ethanol fermentation characteristics of Pichia stipitis yeast from sugar beet pulp hydrolysate: use of new detoxification methods. Fuel. 158, 793–799 (2015). doi:10.1016/j.fuel.2015.06.016
Nilvebrant, N.-O., Persson, P., Reimann, A., De Sousa, F., Gorton, L., Jönsson, L.J.: Limits for alkaline detoxification of dilute-acid lignocellulose hydrolysates. Appl. Biochem. Biotechnol. 105–108, 615–628 (2003)
Martinez, A., Rodriguez, M.E., York, S.W., Preston, J.F., Ingram, L.O.: Effects of Ca(OH)2 treatments (“overliming”) on the composition and toxicity of bagasse hemicellulose hydrolysates. Biotechnol. Bioeng. 69, 526–536 (2000)
Jönsson, L.J., Alriksson, B., Nilvebrant, N.-O.: Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol. Biofuels. 6, 16 (2013). doi:10.1186/1754-6834-6-16
Díaz, M.J., Ruiz, E., Romero, I., Cara, C., Moya, M., Castro, E.: Inhibition of Pichia stipitis fermentation of hydrolysates from olive tree cuttings. World J. Microbiol. Biotechnol. 25, 891–899 (2009). doi:10.1007/s11274-009-9966-9
Palmqvist, E., Hahn-Hägerdal, B.: Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour. Technol. 74, 17–24 (2000). doi:10.1016/S0960-8524(99)00160-1
Huang, C.-F., Lin, T.-H., Guo, G.-L., Hwang, W.-S.: Enhanced ethanol production by fermentation of rice straw hydrolysate without detoxification using a newly adapted strain of Pichia stipitis. Bioresour. Technol. 100, 3914–3920 (2009). doi:10.1016/j.biortech.2009.02.064
Acknowledgements
This work was supported by scholarships and financial assistance for research and development provided by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). The strains of microorganisms utilized in this work were kindly given by Professor Thomas W. Jeffries of the University of Wisconsin. The palm press fiber was donated by the Agropalma, PA, Brazil.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brito, P.L., de Azevedo Ferreira, C.M., Silva, A.F.F. et al. Hydrolysis, Detoxification and Alcoholic Fermentation of Hemicellulose Fraction from Palm Press Fiber. Waste Biomass Valor 9, 957–968 (2018). https://doi.org/10.1007/s12649-017-9882-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9882-4