Abstract
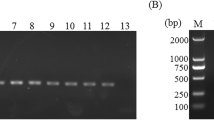
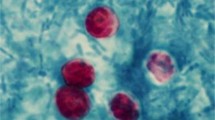
The purpose of this study was to optimize primary and nested polymerase chain reaction (PCR) assays for detecting the microsporidia Encephalitozoon intestinalis and Enterocytozoon bieneusi in fecal samples from dairy calves. PCR for these microsporidia were compared to immunofluorescence assays (IFA) based on commercially available monoclonal antibodies specific for outer wall proteins of Enc. intestinalis or Ent. bieneusi. Fecal samples were collected from 15 dairy calves and processed by molecular sieving followed by salt floatation to recover Enc. intestinalis and Ent. bieneusi spores. An aliquot of the final supernatant was applied to glass slides for IFA testing; another aliquot was extracted for total DNA using a QIAamp Stool Mini-Kit for primary and nested Enc. intestinalis- and Ent. bieneusi-specific PCR analysis. Internal standards were generated for both Enc. intestinalis and Ent. bieneusi PCR assays to control for false negative reactions due to the presence of inhibitors commonly found in fecal samples. Using the commercial MicrosporIFA (Waterborne, Inc.) as the gold standard, the optimized Enc. intestinalis PCR method provided 85.7% sensitivity and 100% specificity with a kappa value = 0.865. Likewise, using the commercial BienusiGlo IFA (Waterborne, Inc.) as the gold standard, the optimized Ent. bieneusi PCR method provided 83.3% sensitivity and 100% specificity with a kappa value = 0.857. Sequencing of amplicons from both PCR assays confirmed the presence of Enc. intestinalis or Ent. bieneusi. In conclusion, our optimized assays for recovering and detecting Enc. intestinalis or Ent. bieneusi in feces from dairy calves provides a valuable alternative to traditional IFA methods that require expertise to identify extremely small microsporidia spores (~ 2.0 µm). Our assays also improve upon existing molecular detection techniques for these microsporidia by incorporating an internal standard to control for false negative reactions.


Similar content being viewed by others
References
Alfa Cisse O, Ouattara A, Thellier M, Accoceberry I, Biligui S, Minta D, Doumbo O, Desportes-Livage I, Thera MA, Danis M, Datry A (2002) Evaluation of an immunofluorescent-antibody test using monoclonal antibodies directed against Enterocytozoon bieneusi and Encephalitozoon intestinalis for diagnosis of intestinal microsporidiosis in Bamako (Mali). J Clin Microbiol 40:1715–1718
Barbosa J, Rodrigues AG, Pina-Vaz C (2009) Cytometric approach for detection of Encephalitozoon intestinalis, an emergent agent. Clin Vaccine Immunol 6:1021–1024
Beckers PJ, Derks GJ, Gool T, Rietveld FJ, Sauerwein RW (1996) Encephalocytozoon intestinalis-specific monoclonal antibodies for laboratory diagnosis of microsporidiosis. J Clin Microbiol 34:282–285
Bergallo M, Costa C, Tarallo S, Daniele R, Merlino C, Segoloni GP, Negro Ponzi A, Cavallo R (2006) Development of a quantitative-competitive PCR for quantification of human cytomegalovirus load and comparison with antigenaemia, viraemia and pp67 RNA detection by nucleic acid sequence-based amplification. Panminerva Med 48:119–127
Buckholt MA, Lee JH, Tzipori S (2002) Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl Environ Microbiol 68:2595–2599
David F, Schuitema AR, Sarfati C, Liguory O, Hartskeerl RA, Derouin F, Molina JM (1996) Detection and species identification of intestinal microsporidia by polymerase chain reaction in duodenal biopsies from human immunodeficiency virus-infected patients. J Infect Dis 174:874–877
Didier ES (2005) Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop 94:61–76
Dowd SE, John D, Eliopolus J, Gerba CP, Naranjo J, Klein R, López B, de Mejía M, Mendoza CE, Pepper IL (2003) Confirmed detection of Cyclospora cayetanesis, Encephalitozoon intestinalis and Cryptosporidium parvum in water used for drinking. J Water Health 1:117–123
Enriquez FJ, Ditrich O, Palting JD, Smith K (1997) Simple diagnosis of Encephalitozoon sp. microsporidial infections by using a panspecific antiexospore monoclonal antibody. J Clin Microbiol 35:724–729
Fedorko DP, Nelson NA, Cartwright CP (1995) Identification of microsporidia in stool specimens by using PCR and restriction endonucleases. J Clin Microbiol 33:1739–1741
Ghoshal U, Khanduja S, Pant P, Ghoshal UC (2016) Evaluation of immunoflourescence antibody assay for the detection of Enterocytozoon bieneusi and Encephalitozoon intestinalis. Parasitol Res 115:3709–3713
Han B, Weiss LM (2017) Microsporidia: obligate intracellular pathogens within the fungal kingdom. Microbiol Spectr 5:1–17
Hanahan D (1983) Studies on the transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hawash Y, Ghonaim MM, Al-Hazmi AS (2015) Internal amplification control for a Cryptosporidium diagnostic PCR: construction and clinical evaluation. Korean J Parasitol 53:147–154
Hoffman RM, Wolk DM, Spencer SK, Borchardt MA (2007) Development of a method for the detection of waterborne microsporidia. J Microbiol Methods 70:312–318
Izquierdo F, Castro-Hermida JA, Fenoy S, Mezo M, González-Warleta M, del Aguila C (2011) Detection of microsporidia in drinking water, wastewater and recreational rivers. Water Res 45:4837–4843
Jenkins MC, O’Brien CN, Santin M, Fayer R (2015) Changes in the levels of Cryspovirus during in vitro development of Cryptosporidium parvum. Parasitol Res 114:2063–2068
Katzwinkel-Wladarsch S, Lieb M, Helse W, Löscher T, Rinder H (1996) Direct amplification and species determination of microsporidian DNA from stool specimens. Trop Med Int Health 1:373–378
Katzwinkel-Wladarsch S, Deplazes P, Weber R, Löscher T, Rinder H (1997) Comparison of polymerase chain reaction with light microscopy for detection of microsporidia in clinical specimens. Eur J Clin Microbiol Infect Dis 16:7–10
Lallo MA, Vidoto Da Costa LF, Alvares-Saraiva AM, Rocha PR, Spadacci-Morena DD, Konno FT, Suffredini IB (2015) Culture and propagation of microsporidia of veterinary interest. J Vet Med Sci 78:171–176
Li X, Tate KW, Dunbar LA, Huang B, Atwill ER (2003) Efficiency for recovering Encephalitozoon intestinalis spores from waters by centrifugation and immunofluorescence microscopy. J Eukaryot Microbiol 50:579–580
Moura H, Sodre FC, Bornay-Llinares FJ, Leitch GJ, Navin T, Wahlquist S, Bryan R, Meseguer I, Visvesvara GS (1999) Detection by an immunofluorescence test of Encephalitozoon intestinalis spores in routinely formalin-fixed stool samples stored at room temperature. J Clin Microbiol 37:2317–2322
Müller A, Stellermann K, Hartmann P, Schrappe M, Fätkenheuer G, Salzberger B, Diehl V, Franzen C (1999) A powerful DNA extraction method and PCR for detection of microsporidia in clinical stool specimens. Clin Diagn Lab Immunol 6:243–246
Musiani M, Gallinella G, Venturoli S, Zerbini M (2007) Competitive PCR-ELISA protocols for the quantitative and the standardized detection of viral genomes. Nat Protoc 2:2511–2519
Notermans DW, Peek R, de Jong MD, Wentink-Bonnema EM, Boom R, van Gool T (2005) Detection and identification of Enterocytozoon bieneusi and Encephalitozoon species in stool and urine specimens by PCR and differential hybridization. J Clin Microbiol 43:610–614
Piña-Vázquez C, Saavedra R, Hérion P (2008) A quantitative competitive PCR method to determine the parasite load in the brain of Toxoplasma gondii-infected mice. Parasitol Int 57:347–353
Ross R, Kleinz R, Reske-Kunz AB (1995) A method for rapid generation of competitive standard molecules for RT-PCR avoiding the problem of competitor/probe cross-reactions. PCR Methods Appl 4:371–375
Saigal K, Khurana S, Sharma A, Sehgal R, Malla N (2013) Comparison of staining techniques and multiplex next PCR for diagnosis of intestinal microsporidiosis. Diagn Microbiol Infect Dis 77:248–249
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Stentiford GD, Becnel J, Weiss LM, Keeling PJ, Didier ES, Williams BP, Bjornson S, Kent ML, Freeman MA, Brown MJF, Troemel ER, Roesel K, Sokolova Y, Snowden KF, Solter L (2016) Microsporidia—emergent pathogens in the global food chain. Trends Parasitol 32:336–348
Watson PF, Petrie A (2010) Method agreement analysis: a review of correct methodology. Theriogenology 73:1167–1179
Wolk DM, Schneider SK, Wengenack NL, Sloan LM, Rosenblatt JE (2002) Real-time PCR method for detection of Encephalitozoon intestinalis from stool specimens. J Clin Microbiol 40:3922–3928
Acknowledgements
The authors acknowledge helpful advice from Monica Santin on microsporidia detection by PCR. This project was solely funded by the USDA-ARS CRIS project “Zoonotic Parasites Affecting Food Animals, Food Safety, and Public Health” (Project No. 8042-32000-100-00-D).
Author information
Authors and Affiliations
Contributions
MJ designed the experiments, performed the IFA testing of isolated microsporidia, and did statistical analyses of the recovery data. CO performed the cell culture for Enc. intestinalis purification, collected, processed and isolated microsporidian spores from calf feces. CP developed the internal standards, extracted DNA and performed the PCR assays to detect purified microsporidia spores in calf fecal samples.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Jenkins, M.C., O’Brien, C.N. & Parker, C. An optimized assay for detecting Encephalitozoon intestinalis and Enterocytozoon bieneusi in dairy calf feces using polymerase chain reaction technology. J Parasit Dis 43, 75–82 (2019). https://doi.org/10.1007/s12639-018-1060-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-018-1060-5