Abstract
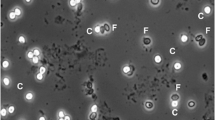
To determine the sensitivity and specificity of routine screening methods for cryptosporidiosis, three methods including conventional modified Ziehl–Neelsen (MZN), direct fluorescent-antibody (DFA) and Nested-PCR assay compared together. To this end, their ability to identify the low concentrations of Cryptosporidium spp. oocysts in children fecal samples was evaluated. The sample population of this study was children under 12 years old who had diarrhea and referred to pediatric hospitals in Tehran, Iran. 2,510 stool specimens from patients with diarrhea were screened for Cryptosporidium oocysts by concentration method and MZN. To determine sensitivity and specificity, Nested-PCR and DFA were performed on 30 positive and 114 negative samples which previously had been proved by MZN. By using the microscopic method, DFA assay and PCR analysis, a total of 30 (1.2 %), 28 (1.1 %) and 32 (1.27 %) positive samples were detected respectively. According to the results, the sensitivity, specificity, and positive and negative predictive values of the Nested-PCR assay were 100 %, compared to 94, 100, 100, and 98 %, respectively, for MZN and 87.5, 100, 100, and 96 %, respectively, for DFA. Results of the present study showed that the Nested-PCR assay was more sensitive than the other two methods and laboratories can use the Nested-PCR method for precise diagnosis of Cryptosporidium spp. However, regarding the costs of Nested-PCR and its unavailability in all laboratories and hospitals, MZN staining on smears has also enough accuracy for Cryptosporidium diagnosis.




Similar content being viewed by others
References
Barugahare R, Dennis MM, Becker JA, Sˇlapeta J (2011) Detection of Cryptosporidium molnari oocysts from fish by fluorescent-antibody staining assays for Cryptosporidium spp. affecting humans. App Environ Microbiol 77:1878–1880
Barwick RS, Levy DA, Craun GF, Beach MJ, Calderon RL (2000) Surveillance for waterborne-disease outbreaks-United States, 1997–1998. MMWR Morb Mortal Wkly Rep 49:1–21
Bialeka R, Bindera N, Dietzb K, Joachimc A, Knobloch J, Zelcka UE (2002) Comparison of fluorescence, antigen and PCR assays to detect Cryptosporidium parvum in fecal specimens. Diagn Microbiol Infect Dis 43:283–288
Cole DJ (1997) Detection of Cryptosporidium parvum using the Kinyoun acid-fast stain. Proc Annu Conv AAEP 43:409–410
Fayer R, Ungar BL (1986) Cryptosporidium spp. and cryptosporidiosis. Microbiol Rev 50:458–483
Fayer R, Xiao L (2008) Cryptosporidium and cryptosporidiosis, 2nd edn. CRC, Boca Raton
Fayer R, Morgan U, Upton SJ (2000) Epidemiology of Cryptosporidium: transmission, detection and identification. Int J Parasitol 30:1305–1322
Garcia LS, Shimizu RY (1997) Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol 35:1526–1529
Garcia LS, Brewer TC, Bruckner DA (1987) Fluorescence detection of Cryptosporidium oocysts in human fecal specimens by using monoclonal antibodies. J Clin Microbiol 25:119–121
Garcia LS, Shum AC, Bruckner DA (1992) Evaluation of a new monoclonal antibody combination reagent for direct fluorescence detection of Giardia cysts and Cryptosporidium oocysts in human fecal specimens. J Clin Microbiol 30:3255–3257
Kaushik K, Khurana S, Wanchu A, Malla N (2008) Evaluation of staining techniques, antigen detection and nested PCR for the diagnosis of cryptosporidiosis in HIV seropositive and seronegative patients. Parasitol Res 107:1–7
Lumb R, Erlich J, Davidson GP (1985) Cryptosporidia detection. Med J Aust 142:329–330
McDonald V (1996) Cryptosporidiosis. In: Cox FEG (ed) Welcome trust illustrated history of tropical disease. The Welcome Trust, London, pp 257–2565
Morgan UM, Constantine CC, Forbes DA, Thompson RCA (1997) Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J Parasitol 83:825–830
Morgan UM, Pallant L, Dwyer BW, Forbes DA, Rich G, Thompson RCA (1998) Comparision of PCR and microscopy for detection of Cryptosporidium parvum in human fecal Specimens. J Clin Microbiol 36:995–998
Paul S, Chandra D, Tewari AK, Banerjee PS, Ray DD, Boral R (2009) Comparative evaluation and economic assessment of coprological diagnostic methods and PCR for detection of Cryptosporidium spp. in bovines. Vet Parasitol 164:291–295
Scheffler EH, VanEtta LL (1994) Evaluation of rapid commercial enzyme immunoassay for detection of Giardia lamblia in formalin-preserved stool specimens. J Clin Microbiol 32:1807–1808
Sterling CR, Arrowood MJ (1986) Detection of Cryptosporidium spp. infection using a direct immunofluorescent assay. Ped infect dis 5:139–142
Vohra P, Singla P, Sharma M, Yadav A, Chaudhary U (2012) Comparison of direct Immunofluorescence, iodine-saline wet mount and modified acid fast staining methods for detection of Cryptosporidium and Giardia spp. in human fecal specimens. JEMDS 1:285–289
Xiao L, Bern C, Limor J, Sulaiman I, Roberts J, Checkley W et al (2001) Identification five types of Cryptosporidium parasites in children in Lima, Peru. J Infect Dis 183:492–497
Zaidah AR, Chan YY, SitiAsma H, Shukri A, Nurhaslindawati A, Salleh M, Zeehaida M, Lalitha P, Mustafa M, Ravichandran M (2008) Detection of Cryptosporidium parvum in HIV-infected patients in Malaysia using a molecular approach. South Asian J Trop Med Pub Health 39:140–151
Ziegler PE, Santucci F, Lindergard G, Nydam DV, Wade SE, Schaaf SL, Chang YF, Mohammed HO (2007) Evaluation of polymerase chain reaction diagnosis of Cryptosporidium spp. in dairy cattle and wildlife. Vet Ther 8:148–159
Zimmerman SK, Needham CA (1995) Comparison of conventional stool concentration and preserved-smear methods with Merifluor Cryptosporidium/Giardia direct immunofluorescence assay and ProSpecT Giardia EZ microplate assay for detection of Giardia lamblia. J Clin Microbiol 33:1942–1943
Acknowledgments
This research was part of master’s (MSc) thesis and financially supported by Vice Chancellors for Education of Shahid Beheshti University of Medical Sciences and approved by Ethical Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran. The authors wish to thank the Shahid Fahmideh, Mofid pediatric hospitals and Mahak Medical Center staffs in Tehran city, for their great assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghamolaie, S., Rostami, A., Fallahi, S. et al. Evaluation of modified Ziehl–Neelsen, direct fluorescent-antibody and PCR assay for detection of Cryptosporidium spp. in children faecal specimens. J Parasit Dis 40, 958–963 (2016). https://doi.org/10.1007/s12639-014-0614-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-014-0614-4