Abstract
Bisfunctional dichlorosilanes were reacted with tetrasilanol of double-decker shaped octaphenylsilsesquioxane (DDSQ) to form fully condensed DDSQ compounds. The crystallographic and thermal characteristics of these compounds were examined. For compounds capped with dichlorosilanes bearing linear aliphatic moieties, as the number of carbon increases from methyl to n-butyl (from 1 to 4) the melting temperature, Tm, dropped from 546 K to 416 K. While for compounds capped with cycloaliphatic, as the moiety changes from cyclopentyl to cyclohexyl, the value of Tm increases from 533 K to 555 K. Surprising, the highest Tm observed was that when capping was done using diisopropyl dichlorosilane. A Tm of around 565 K was observed, which was even higher as compare to diphenyl dichlorosilane capped DDSQ, which had Tm of about 526 K. Phase behavior of binary and ternary mixtures of these condensed DDSQ was also investigated. To our surprise, mixtures of these compounds form eutectic. The eutectic points were calculated based on ideal binary and ternary eutectic from the thermal properties of pure components. Depending on the crystallography, experimental observations of eutectic match well with calculated values.
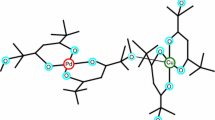
Graphical abstract

Melting and evaporation temperatures were identified for functionalized double-decker shaped silsesquioxanes, melting temperature suppression was observed after mixing DDSQ with different functional groups.
Similar content being viewed by others
Data Availability
Materials and equipment used for this work are described within the manuscript. In addition, experimental details, spectral information, crystal structures, DSC traces, and phase diagrams are also provided in supplementary information.
References
Schoen BW, Holmes D, Lee A (2013) Identification and quantification of cis and trans isomers in aminophenyl double-decker silsesquioxanes using 1H-29Si gHMBC NMR. Magn Reson Chem 51:490–496. https://doi.org/10.1002/mrc.3962
Schoen BW, Lira CT, Lee A (2014) Separation and solubility of Cis and trans isomers in nanostructured double-Decker Silsequioxanes. J Chem Eng Data 59:1483–1493. https://doi.org/10.1021/je4010245
Seurer B, Vij V, Haddad T, Mabry JM, Lee A (2010) Thermal transitions and reaction kinetics of Polyhederal Silsesquioxane containing Phenylethynylphthalimides. Macromolecules 43:9337–9347. https://doi.org/10.1021/ma101640q
Moore LMJ, Zavala JJ, Lamb JT, Reams JT, Yandek GR, Guenthner AJ, Haddad TS, Ghiassi KB (2018) Bis-phenylethynyl polyhedral oligomeric silsesquioxanes: new high-temperature, processable thermosetting materials. RSC Adv 8:27400–27405. https://doi.org/10.1039/C8RA05954C
Hoque MA, Kakihana Y, Shinke S, Kawakami Y (2009) Polysiloxanes with periodically distributed isomeric double-Decker Silsesquioxane in the Main chain. Macromolecules 42:3309–3315. https://doi.org/10.1021/ma900124x
Wei K, Wang L, Zheng S (2013) Organic–inorganic polyurethanes with 3,13-dihydroxypropyloctaphenyl double-decker silsesquioxane chain extender. Polym Chem 4:1491–1501. https://doi.org/10.1039/C2PY20930F
Tanaka T, Hasegawa Y, Kawamori T, Kunthom R, Takeda N, Unno M (2019) Synthesis of double-Decker Silsesquioxanes from substituted Difluorosilane. Organometallics 38:743–747. https://doi.org/10.1021/acs.organomet.8b00896
Mituła K, Dudziec B, Marciniec B (2018) Synthesis of Dialkenyl-substituted double-Decker Silsesquioxanes as precursors for linear Copolymeric systems. J Inorg Organomet Polym Mater 28:500–507. https://doi.org/10.1007/s10904-017-0746-y
Walczak M, Januszewski R, Majchrzak M, Kubicki M, Dudziec B, Marciniec B (2017) Unusual cis and trans architecture of dihydrofunctional double-decker shaped silsesquioxane and synthesis of its ethyl bridged π-conjugated arene derivatives. New J Chem 41:3290–3296. https://doi.org/10.1039/C7NJ00255F
Żak P, Dudziec B, Dutkiewicz M, Ludwiczak M, Marciniec B, Nowicki M (2016) A new class of stereoregular vinylene-arylene copolymers with double-decker silsesquioxane in the main chain. J Polym Sci Part A: Polym Chem 54:1044–1055. https://doi.org/10.1002/pola.27957
Wang M, Chi H, KS J, Wang F (2019) Progress in the synthesis of Bifunctionalized polyhedral oligomeric Silsesquioxane. Polymers 11:2098. https://doi.org/10.3390/polym11122098
Beata D, Bogdan M (2017) Double-decker silsesquioxanes: current chemistry and applications. Curr Org Chem 21:2794–2813
Cao J, Fan H, Li B-G, Zhu S (2017) Synthesis and evaluation of double-Decker Silsesquioxanes as modifying agent for epoxy resin. Polymer 124:157–167. https://doi.org/10.1016/j.polymer.2017.07.056
Xu S, Zhao B, Wei K, Zheng S (2018) Organic-inorganic polyurethanes with double decker silsesquioxanes in the main chains: morphologies, surface hydrophobicity, and shape memory properties. J Polym Sci B Polym Phys 56:893–906. https://doi.org/10.1002/polb.24603
Liu N, Wei K, Wang L, Zheng S (2016) Organic–inorganic polyimides with double decker silsesquioxane in the main chains. Polym Chem 7:1158–1167. https://doi.org/10.1039/c5py01827g
Liu N, Li L, Wang L, Zheng S (2017) Organic-inorganic polybenzoxazine copolymers with double decker silsesquioxanes in the main chains: synthesis and thermally activated ring-opening polymerization behavior. Polymer 109:254–265. https://doi.org/10.1016/j.polymer.2016.12.049
Wu S, Hayakawa T, Kakimoto M-A, Oikawa H (2008) Synthesis and characterization of Organosoluble aromatic polyimides containing POSS in Main chain derived from double-Decker-shaped Silsesquioxane. Macromolecules 41:3481–3487. https://doi.org/10.1021/ma7027227
Jiang Q, Zhang W, Hao J, Wei Y, Mu J, Jiang Z (2015) A unique “cage–cage” shaped hydrophobic fluoropolymer film derived from a novel double-decker structural POSS with a low dielectric constant. J Mater Chem 3:11729–11734. https://doi.org/10.1039/C5TC02432C
Wang Z, Yonggang L, Huijuan M, Wenpeng S, Tao L, Cuifen L, Junqi N, Guichun Y, Zuxing C (2018) Novel phosphorus-nitrogen-silicon copolymers with double-decker silsesquioxane in the main chain and their flame retardancy application in PC/ABS. Fire Mater 2:16230–16957. https://doi.org/10.1002/fam.2649
Vogelsang DF, Maleczka RE, Lee A (2019) Predictive liquid chromatography separation for mixtures of functionalized double-Decker shaped Silsesquioxanes based on HPLC chromatograms. Ind Eng Chem Res 58:403–410. https://doi.org/10.1021/acs.iecr.8b05490
Vogelsang DF, Dannatt JE, Schoen BW (2019) Phase behavior of cis-trans mixtures of double-decker shaped Silsesquioxanes for Processability enhancement. ACS App Nano 2:1223–1231. https://doi.org/10.1021/acsanm.8b02114
Vogelsang DF, Maleczka RE, Lee A (2019) HPLC characterization of cis and trans mixtures of double-Decker shaped Silsesquioxanes. Silicon Chem 11:5–13. https://doi.org/10.1007/s12633-018-0045-4
Clavaguera N, Saurina J, Lheritier J, Masse J, Chauvet A, Clavaguera-Mora MT (1997) Eutectic mixtures for pharmaceutical applications: a thermodynamic and kinetic study. Thermochim Acta 290:173–180. https://doi.org/10.1016/S0040-6031(96)03077-8
Fiala S, Brown MB, Jones SA (2011) Dynamic in-situ eutectic formation for topical drug delivery. J Pharm Pharmacol 63:1428–1436
Lee A, Lu Y, Roche A, Pan T-Y (2016) Influence of Nano-structured Silanols on the microstructure and mechanical properties of A4047 and A359 aluminum casting alloys. Int J Met 10:338–341. https://doi.org/10.1007/s40962-016-0044-4
Liu S, Ma L, Shu Y, Subramanian KN, Lee A, Guo F (2014) Effects of POSS-Silanol addition on whisker formation in Sn-based Pb-free electronic solders. J Electron Mater 43:26–32. https://doi.org/10.1007/s11664-013-2672-2
Brostow W, Goodman SH, Wahrmund J (2013) In: Dodiuk H and Goodman SH (ed) Handbook of Thermoset Plastics, 3rd edn. Elsevier, Oxford, UK
Fina A, Tabuani D, Carniato F, Frache A, Boccaleri E, Camino G (2006) Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim Acta 440:36–42. https://doi.org/10.1016/j.tca.2005.10.006
Blanco I, Abate L, Bottino FA (2017) Mono substituted octaphenyl POSSs: the effects of substituents on thermal properties and solubility. Thermochim Acta 655:117–123. https://doi.org/10.1016/j.tca.2017.06.019
Acknowledgments
Authors are grateful to Drs. Daniel Holmes, Richard Staples and Ms. Emily Dong for their support of NMR, X-ray crystallography and DSC studies, respectively. Additionally, Dr. Jairo Perilla from Universidad Nacional of Colombia for his collaboration and valuable discussion.
Code Availability
Not applicable.
Funding
This work was partially supported by the Office of Naval Research (N00014–16-2109) for generous funding.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding authors
Ethics declarations
Conflicts of Interest/Competing Interests
All authors declare there are no conflicts or competing interests.
Ethics Approval and Consent to Participate
The work described is original and has not been published before in any form of language; it is not under consideration for publication elsewhere; all authors had reviewed and approved the manuscript and supplementary information.
Consent to Publication
Yes
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 10589 kb)
Rights and permissions
About this article
Cite this article
Vogelsang, D.F., Maleczka, R.E. & Lee, A. Phase Behavior of Selected Condensed Double-Decker Shaped Silsesquioxane Compounds. Silicon 14, 7555–7565 (2022). https://doi.org/10.1007/s12633-021-01470-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-021-01470-0