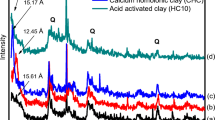
Abstract
The synthesis of a copper(II) oxide–montmorillonite composite and its application in the removal of lead(II) ions in solution were investigated. The Acros Organics (ACOR) montmorillonite was activated using potassium hydroxide solution. The activated ACOR montmorillonite was titrated with copper(II) nitrate solution to produce the copper(II) oxide–montmorillonite composite. Adsorption experiments were conducted using batch-mode techniques under reducing conditions at ambient temperature. The reaction mechanism indicated a higher proton coefficient, greater intraparticle diffusion, and higher mass transfer rates compared with those achieved with bare montmorillonite. The intraparticle diffusion constant derived from the slope was 2.93−3 (mg⋅g−1⋅min−0.5), and the intercept C was 9.86, ≠ 0. In the presence of a CuO coating, the adsorption efficiency was 85.55% at pH 4 and 89.62% at pH 7. Therefore, the copper(II) oxide-montmorillonite composite, as a novel adsorbent with a very high adsorption capacity, exhibited substantially enhanced adsorption of Pb2+ ions compared with bare montmorillonite.
Similar content being viewed by others

References
M.S. Abdullahi, Toxic effects of lead in humans: an overview, Global Adv. Res. J. Environ. Sci. Toxicol., 2(2013), No. 6, p. 157.
M. Khodadadi, A. Malekpour, and M. Ansaritabar, Removal of Pb (II) and Cu (II) from aqueous solutions by NaA zeolite coated magnetic nanoparticles and optimization of method using experimental design, Microporous Mesoporous Mater., 248(2017) p. 265.
D.R. Tipre and S.R. Dave, Bioleaching process for Cu–Pb–Zn bulk concentrate at high pulp density, Hydrome-tallurgy, 75 (2004) No. 1-4, p. 37.
J. Wan, G.M. Zeng, D.L. Huang, et al., Rhamnolipid stabilized nano-chlorapatite: Synthesis and enhancement effect on Pb- and Cd-immobilization in polluted sediment, J. Hazard. Mater., 343(2018), p. 332.
E.P. Kuncoro, D.R.M. Isnadina, H. Darmokoesoemo, O.R. Fauziah, and H.S. Kusuma, Characterization, kinetic, and isotherm data for adsorption of Pb2+ from aqueous solution by adsorbent from mixture of bagasse-bentonite, Data Brief, 16(2018), p. 622.
H.A. van der Sloot, Systematic leaching behavior of trace elements from construction materials and waste material, Studies Environ. Sci., 48(1991), p. 19.
H.A. Sani, M.B. Ahmad, M.Z. Hussein, N.A. Ibrahim, A. Musa, and T.A. Saleh, Nanocomposite of ZnO with montmo-rillonite for removal of lead and copper ions from aqueous solutions, Process Saf. Environ. Prot., 109(2017), p. 97.
R.R. Pawar, L. Lalhmunsiama, M. Kim, J.G. Kim, S.M. Hong, S.Y. Sawant, and S.M. Lee, Efficient removal of hazardous lead, cadmium, and arsenic fromaqueous environment by iron oxide modified clay-activated carbon composite beads, Appl. Clay Sci., 162(2018), p. 339.
A. Zehhaf, A. Benyoucef, R. Berenguer, C. Quijada, S. Taleb, and E. Morallon, Lead ion adsorption from aqueous solutions in Algerian montmorillonites modified, J. Therm. Anal. Calorim., 110(2012), No. 3, p. 1069
M. Mekhloufi, A. Zehhaf, A. Benyoucef, C. Quijada, and E. Morallon, Removal of 8-quinolinecarboxylic acid herbicide from aqueous solution by adsorption on activated montmorillo-nites, Environ. Monit. Assess., 185(2013), No. 12, p. 10365.
A. Zehhaf, A. Benyoucef, C. Quijada, S. Taleb, and E. Morallón, Algerian natural montmorillonites for arsenic(III) removal in aqueous solution, Int. J. Environ. Sci. Technol., 12(2015), No. 2, p. 595.
A.A. Zaki, M.I. Ahmad, and K.M. Abd El-Rahma, Sorption characteristics of a landfill clay soil as a retardation barrier of some heavy metals, Appl. Clay Sci., 135(2017), p. 167.
H. Qiu, L. Lv, B.C. Pan, Q.J. Zhang, W.M. Zhang, and Q.X. Zhang, Critical review in adsorption kinetic models, J. Zhe-jiang Univ. Sci. A, 10(2009), No. 5, p. 716.
C.S.C. Chiew, H.K. Yeoh, P. Pasbakhsh, K. Krishnaiah, P.E. Poh, B.T. Tey, and E.S. Chan, Halloysite/alginate nanocom-posite beads: Kinetics, equilibrium and mechanism for lead adsorption, Appl. Clay Sci., 119(2016), p. 301.
M. Maruthupandy, Y. Zuo, J.S. Chen, J.M. Song, H.L. Niu, C.J. Mao, S.Y. Zhang, and Y.H. Shen, Synthesis of metal oxide nanoparticles (CuO and ZnO NPs) via biological template and their optical sensor applications, Appl. Surf. Sci., 397(2017), p. 167.
D.L. Wang, Z.F. Lin, T. Wang, Z.F. Yao, M.N. Qin, S.R. Zheng, and W. Lu, Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater., 308(2016), p. 328.
K.G. Akpomie, F.A. Dawodu, and K.O. Adebowale, Mechanism on the sorption of heavy metals from binary-solution by a low-cost montmorillonite and its desorption potential, Alexandria Eng. J., 54(2015), No. 3, p. 757.
M.J. Rwiza, S.Y. Oh, K.W. Kim, and S.D. Kim, Comparative sorption isotherms and removal studies for Pb(II) by physical and thermochemical modification of low-cost agro-wastes from Tanzania, Chemosphere, 195(2018), p. 135.
M.L. Bonnet, D. Costa, E. Protopopoff, and P. Marcus, Theoretical study of the Pb adsorption on Ni, Cr, Fe surfaces and on Ni based alloys, Appl. Surf. Sci., 426(2017), p. 788.
D.E. Egirani and N. Wessey, Effect of clay and goethite mineral systems on lead removal from aqueous solution: Paper II, Asian Acad. Res. J. Multidiscip., 3(2015), No. 4, p. 83.
D. Egirani, N. Wessey, and A. Aderogna, Effect of mineral systems on lead removal from aqueous solution: part I, Asian J. Basic Appl. Sci., 2(2015), No. 2, p. 61.
O. Allahdin, J. Mabingui, M. Warte, and A. Boughriet, Removal of Pb2+ ions from aqueous solutions by fixed-BED column using a modified brick: (Micro)structural, electroki-netic and mechanistic aspects, Appl. Clay Sci., 148(2017), p. 56.
Z.B. Bouabidi, M.H. El-Naas, D. Cortes, and G. McKay, Steel-Making dust as a potential adsorbent for the removal of lead (II) from an aqueous solution, Chem. Eng. J., 334(2018), p. 844.
D.E. Egirani, N.R. Poyi, N. Wessey, Synthesis of zinc oxide-goethite composite and its performance on the adsorption of arsenite in aqueous media, J. Chem. Technol. Appl., 2(2019), No. 2, p. 15.
C. Tournassat, J.A. Davis, C. Chiaberge, S. Grangeon, and I.C. Bour, Modeling the acid–base properties of montmoril-lonite edge surfaces. Environ. Sci. Technol., 50 (2016), p. 13436.
R.S.G. Janaki, K. Sreenivas, and R. Sivasamy, Hyperspectral analysis of clay minerals, Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci., XL-8(2014), p. 443.
E. Eren, Removal of heavy metal ions by Unye (Turkey) bentonite in iron and magnesium oxide-coated Forms, J. Hazard. Mater., 165 (2009), p. 63.
M. Pirveysian and M. Ghiaci, Synthesis and characterization of sulfur functionalized grapheme oxide nanosheets as efficient sorbent for removal of Pb2+, Cd2+, Ni2+ and Zn2+ ions from aqueous solution: A combined thermodynamic and kinetic studies, Appl. Surf. Sci., 428(2018), p. 98.
Q.G. Feng, Q.Y. Lin, F.Z. Gong, S. Sugita, and M. Shoya, Adsorption of lead and mercury by rice husk ash, J. Colloid Interface Sci., 278(2004), No. 1, p. 1.
T.C. Nguyen, P. Loganathan, T.V. Nguyen, S. Vigneswaran, J. Kandasamy, and R. Naidu, Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies, Chem. Eng. J., 270(2015), p. 393.
Acknowledgement
The authors remain grateful to the Niger Delta University for the usual research allowances provided for the running of research projects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egirani, D.E., Poyi, N.R. & Wessey, N. Synthesis of a copper(II) oxide–montmorillonite composite for lead removal. Int J Miner Metall Mater 26, 803–810 (2019). https://doi.org/10.1007/s12613-019-1788-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-019-1788-7