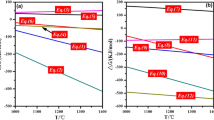
Abstract
To support the development of technology to utilize low-grade Ti–Nb-bearing Fe concentrate, the reduction of the concentrate by coal was systematically investigated in the present paper. A liquid phase formed when the Ti–Nb-bearing Fe concentrate/coal composite pellet was reduced at temperatures greater than 1100°C. The addition of CaCO3 improved the reduction rate when the slag basicity was less than 1.0 and inhibited the formation of the liquid phase. Mechanical milling obviously increased the metallization degree compared with that of the standard pellet when reduced under the same conditions. Evolution of the mineral phase composition and microstructure of the reduced Ti–Nb-bearing Fe concentrate/coal composite pellet at 1100°C were analyzed by X-ray diffraction and scanning electron microscopy–energy-dispersive spectroscopy. The volume shrinkage value of the reduced Ti–Nb-bearing Fe concentrate/coal composite pellet with a basicity of 1.0 was approximately 35.2% when the pellet was reduced at 1100°C for 20 min, which enhanced the external heat transfer to the lower layers when reduced in a practical rotary hearth furnace. The present work provides key parameters and mechanism understanding for the development of carbothermic reduction technology of a Ti–Nb-bearing Fe concentrate incorporated in a pyrometallurgical utilization flow sheet.
Similar content being viewed by others
References
T. Makanyire, A. Jha, and S. Sutcliffe, Kinetics of hydrochloric acid leaching of niobium from TiO2 residues, Int. J. Miner. Process., 157(2016), p. 1.
A. Banu, M. Marcu, S. Petrescu, N. Ionescu, and A. Paraschiv, Effect of niobium alloying level on the oxidation behavior of titanium aluminides at 850°C, Int. J. Miner. Metall. Mater., 23(2016), No. 12, p. 1452.
U.S. Geologica, Mineral Commodity Summaries, U.S. Geological Survey, Reston, 2016, p. 117.
Q.F. Zhang, Analysis on mineralogical characteristics of niobium-bearing resources in Baiyunebo deposit, Nonferrous Met., 57(2005), No. 2, p. 111.
M.D. Liu, Z.X. You, Z.W. Peng, X. Li, and G.H. Li, Enrichment of rare earth and niobium from a REE–Nb–Fe associated ore via reductive roasting followed by magnetic separation, JOM, 68(2016), No. 2, p. 567.
J. Xv, C.K. Bulin, and R.C. Zhao, Recovering iron and beneficiating niobium from rough niobium concentrate by magnetization roast–magnetic separation process, Hydrometallurgy China, 32(2013), No. 2, p. 75.
M. Jiang, T.C. Sun, J. Kou, Y.N. Ji, and Y. Xu, Distribution behavior of niobium in process of coal-based direct reduction roasting of Nb-bearing iron concentrates, Chin. J. Rare Met., 35(2011), No. 5, p. 731.
W. Ma, X. Zong, and Z.Q. He, Reductive behaviour of carbon-bearing pellets of Baotou niobium concentrates, Min. Metall. Eng., 16(1996), No. 2, p. 51.
V.D. Eisenhuttenleute, Slag Atlas, 2nd Ed., Verlag Stahleisen mbH, Düsseldorf, 1995, p. 79.
Y.J. Liang, Y.C. Che, X.Y. Liu, and N.J. Li, Thermodynamic Data Notebook of Inorganics, Northeast University Press, Shenyang, 1993, p. 454.
Y.L. Li, J.H. Yang, and J.S. Gao, Effect of alkali earth metal oxides on kinetic parameters of coke solution loss reaction, J. Fuel Chem. Technol., 29(2001), No. 3, p. 280.
X.M. Yang, Y.S. Xie, D.G. Wang, D.B. Huang, L.T. Kong, and T.J. Yang, Effect of CaO and CaCO3 on reduction rate of iron ore pellets containing carbon, J. Iron Steel Res. Int., 7(2000), No. 2, p. 1.
C. Suryanarayana, Mechanical alloying and milling, Prog. Mater. Sci., 46(2001), No. 1-2, p. 1.
J.V. Khaki, M.R. Aboutalebi, and S. Raygan, The effect of mechanical milling on the carbothermic reduction of hematite, Miner. Process. Extr. Metall. Rev., 25(2004), No. 1, p. 29.
C. Li, B. Liang, L.H. Guo, and Z.B. Wu, Effect of mechanical activation on the dissolution of Panzhihua ilmenite, Miner. Eng., 19(2006), No. 14, p. 1430.
E. Kasai, K. Mae, and F. Saito, Effect of mixed-grinding on reduction process of carbonaceous material and iron oxide composite, ISIJ Int., 35(1995), No. 12, p. 1444.
F. Apaydin, A. Atasoy, and K. Yildiz, Effect of mechanical activation on carbothermal reduction of chromite with graphite, Can. Metall. Q., 50(2011), No. 2, p. 113.
S. Halder and R.J. Fruehan, Reduction of ironoxide-carbon composites: Part III. Shrinkage of composite pellets during reduction, Metall. Mater. Trans. B, 39(2008), No. 6, p. 809.
G. Wang, Q.G. Xue, and J.S. Wang, Volume shrinkage of ludwigite/coal composite pellet during isothermal and non-isothermal reduction, Thermochim. Acta, (2015), No. 621, p. 90.
S. Prakash, Reduction and sintering of fluxed iron ore pellets— a comprehensive review, J. South Afr. Inst. Min. Metall., 96(1995), No. 1, p. 3.
Acknowledgements
The authors would like to express their gratitude for the financial support of the Fundamental Research Funds for the Central Universities (FRF-TP-16-019A1) and the State Key Laboratory of Advanced Metallurgy (41617007), University of Science and Technology Beijing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, G., Du, Yx., Wang, Js. et al. Carbothermic reduction behaviors of Ti–Nb-bearing Fe concentrate from Bayan Obo ore in China. Int J Miner Metall Mater 25, 28–36 (2018). https://doi.org/10.1007/s12613-018-1543-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-018-1543-5