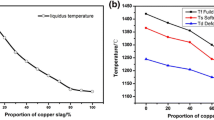
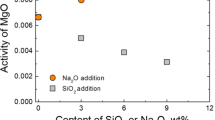
Abstract
Copper smelting slag is a useful secondary resource containing high iron and copper, which can be utilized to prepare crude Fe-Cu alloy by a direct reduction–magnetic separation process for making weathering-resistant steel. However, it is difficult to recover iron and copper from the slag by direct reduction since the iron mainly occurs in fayalite and the copper is held in copper sulfide. Therefore, enhancement reduction of copper slag is conducted to improve the recovery of copper and iron. Additives such as Na2CO3 has been proven to be capable of reinforcing the reduction of refractory iron ore. In this research, the effect of Na2CO3 on the carbothermic reduction of copper slag was investigated, and phase transformations during reduction and the distributing characteristics of iron and copper in the alloy and non-metallic phases of the reduced pellets were also studied. The results show that the metallization rate of iron and copper was increased with the addition of Na2CO3, leading to higher iron and copper recovery in Fe-Cu alloy powder. X-ray diffraction (XRD) and scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS) results confirm that Na2CO3 is capable of enhancing the reduction of fayaltie, copper silicate and copper sulfide, which agrees well with thermodynamic analysis. Furthermore, the reduction mechanism of copper slag was demonstrated based on systematic experimental observations.






Similar content being viewed by others

References
B. Gorai, R. Jana, and M. Premchand, Resour. Conserv. Recycl. 39, 4 (2003).
H. Shen and E. Forssberg, Waste Manag. 23, 10 (2003).
H. Wang, J. Guangdong Nom-ferr. Met. (In China) 13, 4 (2010).
A. Harvey, Resour. Conserv. Recycl. 43, 353 (2005).
J.H. Heo, Y. Chung, and J.H. Park, J. Clean. Prod. 13, 777 (2016).
M. Sánchez and M. Sudbury, J. Min. Metall. B 49, 161 (2013).
I. Muravyov, V. Fomchenko, V. Usoltsev, A. Vasilyev, and F. Kondrateva, Hydrometallurgy 119, 40 (2012).
D. Urosevic, M. Dimitrijevic, Z. Jankovic, and D. Antic, Physicochem. Probl. Min. Process. 51, 71 (2015).
S. Panda, S. Mishra, D.S. Rao, N. Pradhan, U. Mohapatra, S. Angadi, and B.K. Mishra, Korean J. Chem. Eng. 32, 667 (2015).
K. Mawejaa, T. Mukongob, and I. Mutomboc, J. Hazard. Mater. 164, 856 (2009).
W. Bruckard, M. Somerville, and F. Hao, Miner. Eng. 17, 495 (2004).
A. Sarrafi, B. Rahmati, and H.R. Hassani, Miner. Eng. 17, 457 (2004).
Z.Q. Guo, D.Q. Zhu, J. Pan, T.J. Wu, and F. Zhang, Metals 6, 86 (2016).
Z.Q. Guo, D.Q. Zhu, J. Pan, and F. Zhang, JOM 69, 2341 (2016).
Y. Fan, E. Shibata, A. Iizuka, and T. Nakamura, MMTB 46, 2158 (2015).
X.L. Zhou, D.Q. Zhu, J. Pan, and T.J. Wu, Int. ISIJ 55, 1347 (2015).
B. Su, S. Kim, S. Ki Jo, D. Shin, J.C. Lee, and S.B. Jeong, Int. J. Miner. Process. 143, 43–49 (2015).
Z. Zuo, Q. Yu, J. Liu, Q. Qin, H. Xie, F. Yang, and W. Duan, Int. ISIJ. 57, 220 (2017).
B. Chanchal, G. Prithviraj, and D. Arnab, Int. J. Min. Metall. Mater. 12, 1360 (2016).
B. Peters and F. Hoffmann, Chem. Eng. J. 304, 692 (2016).
D.Q. Zhu, Z.Q. Guo, J. Pan, and F. Zhang, Metals 6, 6 (2016).
Acknowledgements
The authors wish to express their thanks to the National Key Technology R&D Program of China (No. 2013BAB03B04) for the financial support of this research, and also would like to thank Co-Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources of Hunan Province, which supplied us the facilities and funds to fulfill the experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, Z., Zhu, D., Pan, J. et al. Effect of Na2CO3 Addition on Carbothermic Reduction of Copper Smelting Slag to Prepare Crude Fe-Cu Alloy. JOM 69, 1688–1695 (2017). https://doi.org/10.1007/s11837-017-2410-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2410-y